Interaction between metallic nanoparticles and biological systems
Published on 25 January 2019
Body text 1
MCT investigator: Giulia Veronesi
Collaborations: LCBM/BioMet team, INAC/SyMMES Grenoble, beamlines ID16B-NA and ID21 of the European Synchrotron Radiation Facility (ESRF)
Metallic nanoparticles (NPs) are introduced in a wide number of consumer goods as healthcare products, cosmetics, food packaging, and confectionery. Their release in the environment and their toxicity to humans are nowadays a matter of concern. The toxicological outcome of metallic NPs is intimately related to the physicochemical transformations they undergo in biological environments, it is therefore crucial to understand the fate of NPs in cells and tissues.
We recently focused on silver nanoparticles (AgNPs): these NPs are used for their bactericidal effect that is due to the release of toxic Ag+ ions from their surface. A drawback is that AgNPs can also enter eukaryotic cells and poison them. Understanding how AgNPs dissolve in cells and what complexes are formed by Ag+ ions is paramount in order to disclose the mechanisms that rule their toxicity. By making use of X-ray Absorption Spectroscopy (XAS) we were able to measure the fraction of silver atoms that were released from AgNPs inside macrophages and hepatocytes; we showed that dissolution rates depend on the NP coating as well as on the exposure mode (acute vs chronic) [1, 2].
We could visualize and quantify Ag species in human hepatocytes (HepG2) exposed to AgNPs by acquiring single-cell X-Ray Fluorescence (XRF) elemental imaging data (Figure) on a newly built synchrotron nanoprobe: the synergistic use of electron microscopy allowed us to propose that AgNPs were located in endocytosis vesicles, while Ag+ ions diffused in the cell [2].
XAS showed that Ag+ ions recombine with thiol-bearing biomolecules both in macrophages and hepatocytes, and allowed us to measure the Ag-S distances in cellulo. This information, corroborated by an exhaustive characterization of Ag+ binding in biological Cu+-thiolate sites [3] led us to propose the involvement of glutathione and metallothioneins in Ag+ binding.
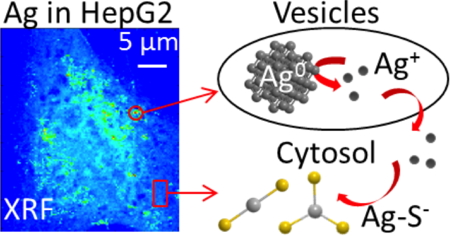
Silver distribution (left) in a HepG2 cell exposed to silver nanoparticles, obtained through nano-beam X-Ray Fluorescence elemental imaging.
Right: proposed fate of nanoparticles and Ag+ ions.
Published in [2].
01 - Veronesi G, Aude-Garcia C, Kieffer I, Gallon T, Delangle P, Herlin-Boime N, Rabilloud T and Carrière M
Exposure-dependent Ag+ release from silver nanoparticles and its complexation in AgS2 sites in primary murine macrophages.
Nanoscale, 2015, 7(16): 7323-7330
02 - Veronesi G, Deniaud A, Gallon T, Jouneau PH, Villanova J, Delangle P, Carrière M, Kieffer I, Charbonnier P, Mintz E and Michaud-Soret I
Visualization, quantification and coordination of Ag+ ions released from silver nanoparticles in hepatocytes.
Nanoscale, 2016, 8(38): 17012-17021
03 - Veronesi G, Gallon T, Deniaud A, Boff B, Gateau C, Lebrun C, Vidaud C, Rollin-Genetet F, Carrière M, Kieffer I, Mintz E, Delangle P and Michaud-Soret I
XAS investigation of silver(I) coordination in copper(I) biological binding sites
Inorganic Chemistry, 2015, 54(24): 11688-11696
Top page