4-hydroxyphenylpyruvate dioxygenase (HPPD) is the molecular target of very effective herbicides. Because of its involvement in the degradation of tyrosine, HPPD inhibitors are also used as therapeutic agents in the fight against the lethal effects associated with tyrosinemia type I. They prevent the accumulation of highly toxic molecules from homogentisate degradation. These inhibitors are also the subject of clinical trials in the treatment of Parkinson's disease by reducing the degradation of tyrosine, a dopamine precursor. Despite its agrochemical and pharmaceutical interest, the reaction mechanism of HPPD has not yet been fully elucidated.
The HPPD catalyzes the conversion of 4-hydroxyphenylpyruvate to homogentisate (HGA). In most organisms, the HPPD is involved in the degradation of tyrosine, but in photosynthetic organisms HGA is the aromatic precursor of plastoquinone and vitamin E, molecules essential for photosynthesis and oxidative stress protection, respectively. In a study based on the 3D structure of the enzyme from Arabidopsis (
1SQD), combining site-directed mutagenesis approach performed in our laboratory and supported by theoretical calculations of quantum mechanics / molecular mechanics (QM/MM) (
Figure), the role of catalytic residues interacting with the substrate and/or reaction intermediates could be determined. These results highlight:

the central role of Q272 Q286 Q358 in the formation of enzyme-substrate complex and the first nucleophilic attack,

the large movement of the aromatic ring of HPP during the reaction,

the key role played by N261 and S246 in the hydroxylation of C 1 and the final rearrangement of the side chain.
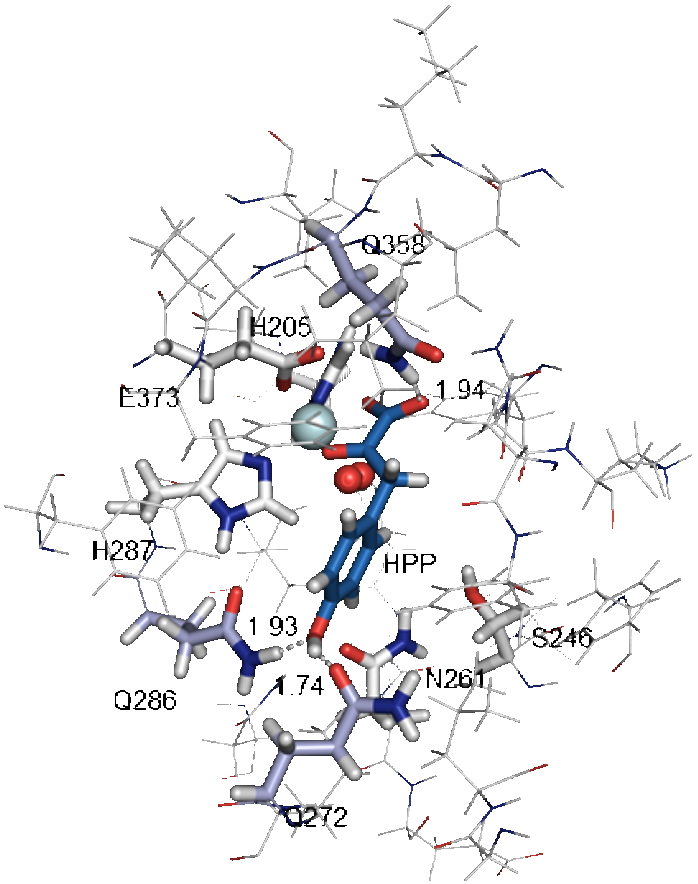
Structure of the ternary complex enzyme-HPP-O2 based on QM / MM theoretical calculations taking into account the results of mutagenesis highlighting the role of residues Q358 Q286 Q272 in the formation of this complex. Iron is in cyan, oxygen in red and HPP in blue.