Nicotinamide adenine dinucleotide (NAD), is an essential and ubiquitous cofactor known for its role as a cosubstrate in a myriad of biological oxidation-reduction reactions. Its biosynthesis involves the formation of quinolinic acid (QA), the biosynthesis of which is different in prokaryotes and eukaryotes. In most eukaryotic organisms, QA is produced by the oxidative degradation of L-tryptophan, whereas in most prokaryotes, QA is generated by a unique condensation reaction between dihydroxyacetone phosphate (DHAP) and iminoaspartate (IA). This reaction requires the concerted actions of two proteins, L-aspartate oxidase (NadB), which first converts L-aspartate into iminoaspartate, and quinolinate synthase (NadA), an iron-sulfur enzyme, which condenses IA with DHAP to form QA. Besides these de novo syntheses of NAD, a salvage pathway exists in some organisms that enables NAD to be recycled from nicotinic acid and nicotinamide (Figure 1). However, some pathogens, such as Mycobacterium leprae, which is the causative agent of leprosy, and Helicobacter pylori, which is a major cause of gastroduodenal diseases, were reported to lack this salvage pathway. The presence in most prokaryotes and eukaryotes of distinct pathways for quinolinic acid biosynthesis, along with the absence of the salvage pathway in some pathogenic microorganisms, makes NadA a novel target for the development of specific antibacterial drugs.
Researchers of our laboratory are interested for unraveling the mechanism of the reaction catalyzed by NadA which so far has been a stumbling block in the elucidation of the de novo quinolinic acid biosynthesis pathway in bacteria.
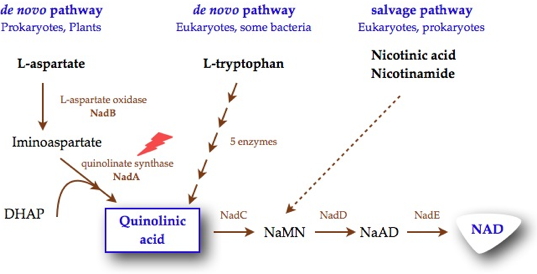
Using 4,5-dithiohydroxyphthalic acid (DTHPA), a structural analogue of a postulated intermediate of the mechanism, these researchers demonstrated that this molecule constitutes an inhibitor of Escherichia coli quinolinate synthase activity in vitro preventing quinolinic acid formation (Figure 2). By a combination of Mössbauer experiments supported by DFT calculations they revealed that DTHPA inhibits NadA by coordination to the NadA Fe4S4 cluster through a differentiated iron site, leading to an inaccessible iron-sulfur cluster for substrates (Figure 3). These results bring to light, for the first time, the existence of a differentiated iron site on the iron-sulfur cluster. The fact that DTHPA binding is associated with the loss of NadA activity in vitro demonstrates also that the chemistry of QA formation, the DHAP and IA condensation, occurs at the Fe4S4 cluster through its differentiated iron (Figure 1).
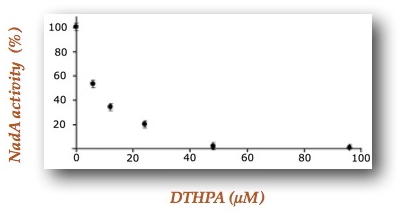
Figure 3: Model for a DTHPA-bound NadA Fe4S4 cluster with protonated carboxylate groups.
Fe atoms in brown, O atoms in red, S atoms in yellow.
These results were also patented [2]. Indeed, DTHPA was shown to be a selective inhibitor of quinolinic acid biosynthesis pathway in vivo on Escherichia coli leading to inhibition of its growth in liquid medium. Investigation of DTHPA acting as an inhibitor of NadA proteins from pathogens such as M. leprae and H. pylori is underway in collaboration with Institut Pasteur and Ecole Polytechnique Fédérale de Lausanne in Switzerland. Indeed, in vivo data on the E. coli strain obtained by these researchers raises the possibility of DTHPA acting as a new antibacterial agent on a new target quinolinate synthase.
Helicobacter pylori is responsible for most of the stomach cancers.
Mycobacterium leprae is a pathogen causing leprosy in human.