Photosynthesis is a fascinating source of inspiration for designing innovative molecular devices, such as dye-sensitized photo-electrochemical cells, for the conversion and storage of solar energy under the form of a fuel. From a fundamental point of view, these devices reproduce the key steps of photosynthesis. Nevertheless, their efficiency is currently limited by an intrinsic property of the system: the electrons are delivered one by one by the photosensitizer, but they are used two by two by the catalyst (case of hydrogen production). Nature overcomes this problem by means of an intermediate step of charge photoaccumulation which allows to temporarily store the reducing equivalents (or oxidants) involved in the reactions of interest. Integrating this process into artificial photosynthetic devices is a challenge for chemists.
Researchers at the Chemistry and Biology of Metals Laboratory (UMR 5249 UGA / CNRS / CEA-Grenoble,
SolHyCat
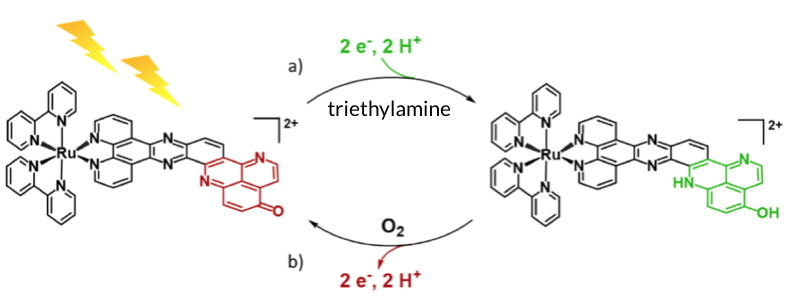
team), of the medicinal chemistry Department (DPM, UMR 5063 UGA / CNRS), of the SyMMES laboratory (UMR 5819 UGA / CNRS / CEA-Grenoble) and the University of Jena (Germany) have designed a new photosensitizer with a charge photoaccumulation site. The electronic properties of this photosensitizer have been studied by different electrochemical and spectroscopic techniques, in combination with DFT calculations. The researchers have demonstrated, under light irradiation and in the presence of an electron source, the reversible storage of two electrons and two protons on the iminobenzoquinone motif of the π-extended ligand (Figure). This storage mechanism is reminiscent of plastoquinones, the electron relays of the photosynthetic chain.
This work, funded by the Arcane Grenoble Labex and the CEA-DRF Impulsion 2017 program, opens new perspectives in bio-inspired photocatalysis for energy.