In order to better understand living processes, biologists need tools that allow them to selectively detect and quantify the molecules that are involved in cellular mechanisms. In this respect, fluorescent probes are particularly useful tools because fluorescence is a very sensitive and inexpensive detection method that is easy to implement in a laboratory.
Researchers from our laboratory have shown that it is possible to design an original protein-based fluorescent probe to detect a short RNA sequence. For this, they have used a recognition domain present on a protein and that is capable of selectively binding the RNA sequence of interest. They were interested in the sequence UUAUUUAUU present in the non-coding region of some messenger RNAs. This 9-base sequence is too short to be detected by conventional RNA probes constructed from DNA.
To overcome this limitation, the researchers took a different approach. They have shown that it is possible to use the recognition domain of tristetraprolin (TTP protein;
Figure) to design a fluorescent probe capable of selectively recognize the target RNA sequence. They have thus synthesized a polypeptide of 68 amino acids corresponding to the domain of the TTP protein that recognizes the UUAUUUAUU sequence. This synthesis carried out chemically allowed them to graft two fluorescent molecules on the polypeptide. The synthetic polypeptide adopts the same folding as the native TTP protein and it changes conformation when it binds to the target RNA sequence as does the native TTP protein. The distance between the two fluorescent molecules is then modulated, which leads to increase the fluorescence emission. In addition, the synthetic probe retains all the recognition properties of the native TTP protein for RNA (affinity, selectivity, binding and stall speeds), properties that are essential for the in vivo use of fluorescent probes for confocal microscopy on cells.
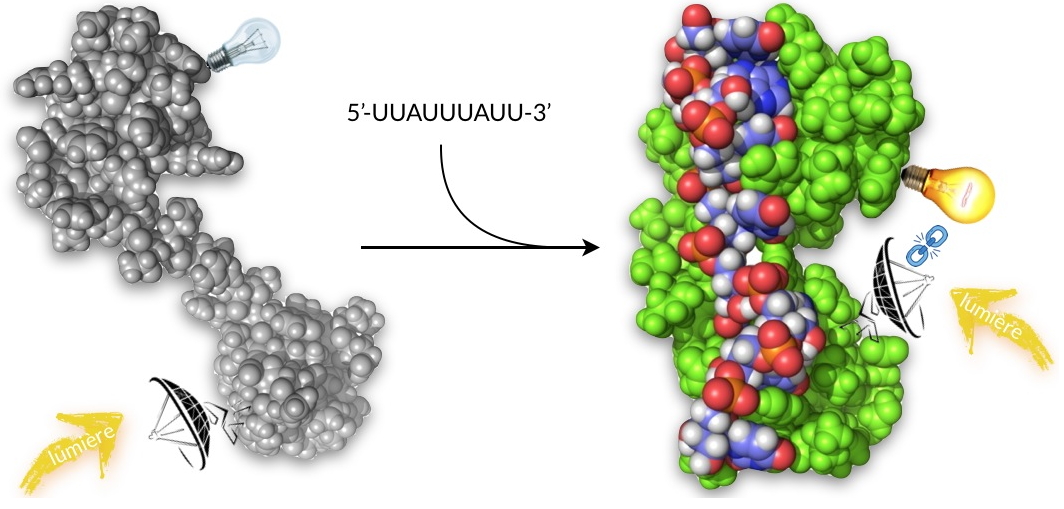
The UUAUUUAUU sequence is found in certain messenger RNAs, for example that of TNF-α, a pro-inflammatory cytokine involved in inflammatory processes or certain cancers. This sequence is the site of binding of the tristetraprolin protein (TTP) which regulates the production of TNF-α by controlling the fate (destruction or translation) of its messenger RNA. The RNA recognition domain of the TTP protein changes conformation by binding to its target, the two fluorescent molecules grafted onto the recognition domain come closer and the probe ignites.