 Leader
Leader
| Dr.
Stéphane Ménage
CNRS research director
Laboratoire Chimie et Biologie des Métaux
CEA-Grenoble
17 avenue des Martyrs
38 054 Grenoble Cedex 09
Phone: (33) 4 38 78 91 03
Fax: (33) 4 38 78 91 24 |
Catalysis is present at all levels of our lives. It is a tool for the creation of all our everyday objects but also in the chemistry of our human body; transforming oil into various plastics or in our food changing milk into yoghurt and promoting your digestion.
Catalysis accelerates a chemical reaction by reducing the energy consumption of the reaction path. It is therefore a pillar of sustainable chemistry since it saves energy and avoids the use of large quantities of harmful products. It involves the development of molecules or assemblies of molecules on a surface based on the understanding of its action at the molecular level. The BioCE team draws its inspiration from living organisms and biocatalysts, enzymes, to build very efficient and stable chemical catalysts to promote sustainable chemistry.
 Our scientific project
Our scientific project The scientific project of “
Bio
in
organic
Catalysis and
Environment” is based on a
bottom-up approach from molecular catalyst to functionalized (bio)materials
via bio-inspired sustainable catalytic processes. Our scientific strategy relies on bioinorganic chemistry, that combines expertise in organic synthesis, inorganic synthesis, physical spectroscopies and analytical chemistry in order to
develop new approaches on bioinspired catalysis. The core of the team project relies then on the design of discrete inorganic complexes, catalysts inspired from the structure of active sites of metalloenzymes, (
i.e. polycyclic aromatic hydrocarbons dioxygenases, nitrous oxide reductase) and the targeted catalysis span from enantioselective oxidations to activation of small molecules. From now, we look towards
heterogeneous catalysis and
synthetic biology for a circular economy in the field of environment, Health and energy,
in order to synthesize chiral drugs, use sustainable catalytic processes and develop new catalysis for energy (CO
2 conversion, O
2 reduction).
 Our strategy
Our strategy The knowledge of the role of metals in biology offers a variety of innovations or scientific advances in the field of synthetic chemistry. First, it may represent a great inspiration to create new molecules or to mimic original complex chemical processes (bio-inspired chemistry). Second, it provides the tools to divert natural processes for the synthesis of new molecules (synthetic biology). In the BioCE team, the strategy based on this knowledge is illustrated in the field of catalysis. If biocatalysis represents the best alternative for a sustainable chemistry, it suffers from a finite repertoire of starting building blocks. Conversely, metal-based chemistry offers a large panel of reactants but is short of complex reactions, such as cascade/domino processes. Then a combination of both catalytic approaches, if controlled, should provide innovative solutions. Keeping this in mind, the BioCE team has been involved in the proof of concept studies for new catalytic strategies based on the design of original inorganic catalysts from oxidation/reduction to oxygen transfer reactions in the last decade. From time to time, the complexity in the structure of the catalytic objects has been raised, with the introduction of new functions or property, in order to gain in catalytic selectivities and efficiency or complexify chemical reactions.
Our approach consists in:
i) deciphering (bio)catalytic mechanisms through the design of chemical mimics;
ii) mimicking the biological organization to perform abiotic reactions;
iii) transferring catalysis from homogeneous to heterogeneous conditions using solid biodegradable materials.
iv) using biodiversity for pollutants degradation.
v) develop biomaterials for new technology for the energies.
Fundamental questions have then been challenged: the mode of action (rationale) for gas activation. (O
2, N
2O), the sulfide over oxygen ligand for copper reactivity, the control of electron transfer during photocatalysis and the molecular aspects of catalytic selectivity for artificial enzymes and the control of self-assembly of biological macromolecules.
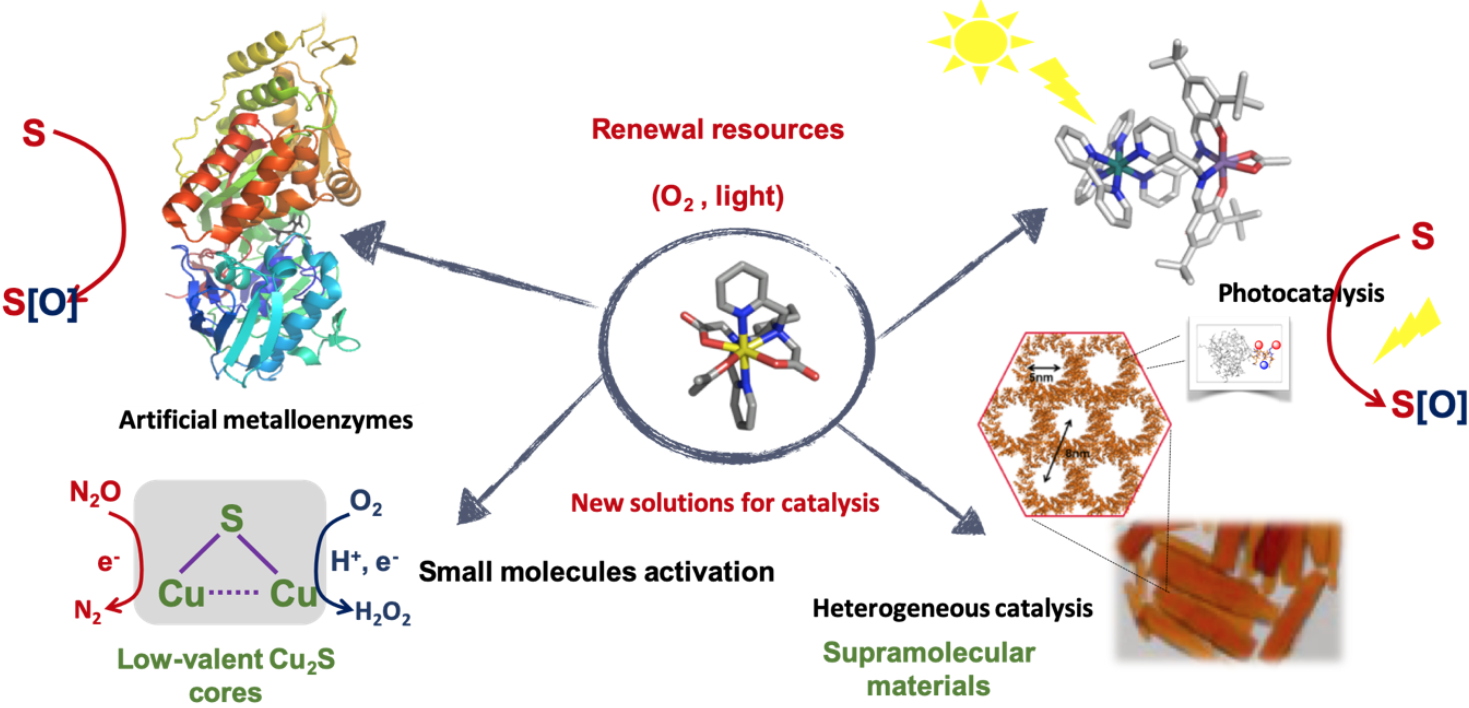
 Our projects
Our projects Then, we decline our scientific expertise in four projects.
1. Design of
artificial metalloenzymes,
2.
Bio-inspired nitrous oxide and dioxygen activation by metal complexes with N/S coordination spheres
3. Development of
photoactivatable catalysts in oxygenation with H
2O as the only source of oxygen.
4.
Enzymatic and chemical degradation of plastics and their valorisation
5.
Macromolecular and supramolecular materials for (bio)electrocatalysis and photo(bio)electrocatalysis