L. Albertin and N. Duraffourg
The aim of this project is to
develop bio-inspired and bio-sourced materials for the (bio)electrochemical and photo(bio)electrochemical conversion of small gas molecules for energy conversion and storage. A particular emphasis will be put on the selective reduction of CO
2 and H
2O to formic acid and H
2, respectively.
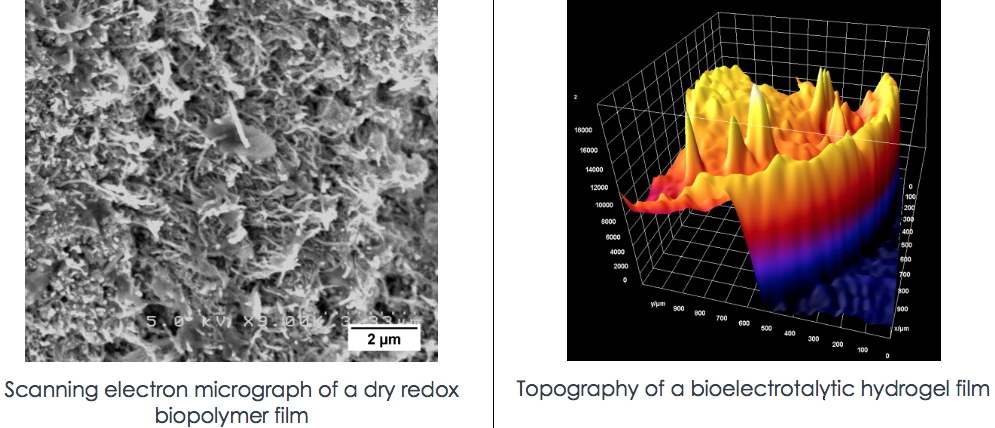
The project rests on the idea that: i) Biocatalysts can efficiently interconvert the oxidized and reduced form of their substrate, but are difficult to connect directly to an electrode and are prone to deactivation under reaction conditions; ii) Incorporation of biocatalysts into a suitable redox hydrogel stabilizes the enzyme within a hydrophilic and solvated environment, and ensures its electrical wiring to the electrode by serving as electron conducting relay. As for artificial molecular catalysts: i)The amount that can be directly wired onto an electrode through adsorption or grafting is limited and depends on the nature of the electrode. ii) Their precise positioning and interfacing onto the electrode is extremely difficult.
We propose to develop modified (bio)electrodes in which: a) Either a suitable enzyme (e.g. formate dehydrogenase, hydrogenase) is entrapped into a biopolymer-based redox matrix; b) Or an artificial molecular (photo)catalyst is rationally integrated into supramolecular nanostructure obtained by protein self-assembly.
In the first case, either chemically modified polysaccharides or protein nano-filaments will be used to enhance the stability of the enzyme while ensuring the necessary electrical wiring. The expression and purification of the enzymes of interest take advantage of the expertise of LCBM in the field of metalloenzymes and molecular biology.
In the second case, the ability of several types of proteins to self-assemble into 1-D and 3-D nanostructures will be exploited: Artificial proteins will be synthesized that can self-assemble into nanostructures displaying both (photo)catalytic sites and organic or inorganic redox relays in an orderly arrangement.