 Amyloidosis is the generic name for diseases in which, normally soluble proteins, accumulate within the extracellular space of various tissues as insoluble deposits of proteins rich in ß-sheet structures, affecting either the central nervous system or a variety of peripheral tissues. Therefore, they are of enormous importance in the context of present-day human health and welfare, but much remains to be learned about the mechanism by which proteins aggregate and form amyloid structures, and how this latter affects biological functions.
Amyloidosis is the generic name for diseases in which, normally soluble proteins, accumulate within the extracellular space of various tissues as insoluble deposits of proteins rich in ß-sheet structures, affecting either the central nervous system or a variety of peripheral tissues. Therefore, they are of enormous importance in the context of present-day human health and welfare, but much remains to be learned about the mechanism by which proteins aggregate and form amyloid structures, and how this latter affects biological functions.
 Nowadays, the diagnosis of amyloidosis disease rests on clinical evaluation combined with histochemical, biochemical and genetic analyses, and sometimes functional imaging studies. Most of the amyloid disorders become visible in imaging only in middle or late life, with a prolonged preclinical phase while proteins are already accumulated compromising progressively cellular and tissue functions.
Nowadays, the diagnosis of amyloidosis disease rests on clinical evaluation combined with histochemical, biochemical and genetic analyses, and sometimes functional imaging studies. Most of the amyloid disorders become visible in imaging only in middle or late life, with a prolonged preclinical phase while proteins are already accumulated compromising progressively cellular and tissue functions.
 Therefore the prognosis is poor, and there is an urgent need for early diagnosis, in order to apply a therapy before irreversible organ alterations.
Therefore the prognosis is poor, and there is an urgent need for early diagnosis, in order to apply a therapy before irreversible organ alterations.
One of our projects consists in the development and the validation of innovative nanoparticles with multifunctional properties for the amyloidosis diagnosis by various imaging methods such as Magnetic Resonance Imagery or PET-Scan. Moreover, specificity of recognition of the amyloid fibrils is targeted by grafting a particular peptide, on the nanoparticles, directed to these proteins (Figure 1). These nanoparticles constitute an multimodal probe challenging detection of amyloid aggregates earlier than with classical diagnosis: both simultaneously MRI and scintigraphy images can be obtained combining the advantages of each technique (i.e. resolution for MRI and sensibility for scintigraphy). Specific grafted-peptides that tagged amyloid fibres would represent an expected inhibitor of the growth of amyloid fibres.
 The first expected results obtain for diabetes type II and TTR amyloidosis will be a proof of concept for the use of nanoparticles in the field of amyloidosis, and similar strategy should be extended to other related diseases such as Alzheimer's and Parkinson's diseases. In the case of success, the expected outputs for human healthcare is important because of the lack of early diagnosis of amyloidosis and constitute an expected extend to effective therapy for stopping their development.
The first expected results obtain for diabetes type II and TTR amyloidosis will be a proof of concept for the use of nanoparticles in the field of amyloidosis, and similar strategy should be extended to other related diseases such as Alzheimer's and Parkinson's diseases. In the case of success, the expected outputs for human healthcare is important because of the lack of early diagnosis of amyloidosis and constitute an expected extend to effective therapy for stopping their development.
 This work is realised in collaboration with several laboratories and supported by European funds.
This work is realised in collaboration with several laboratories and supported by European funds.
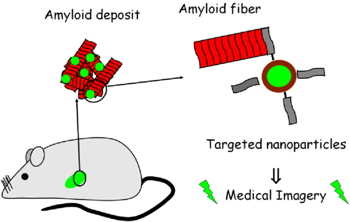
Figure 1.
 Other biological aspects have been associated with the accumulation of misfolded proteins due to various dysfunctions. The proteasome deregulation, because of genetic mutation or oxidative stress, is thought to be also responsible for the accumulation of aggregated proteins within inclusion bodies. Chronic inflammation associated with microglia and mitochondria dysfunctions promote also toxicity and amyloid protein accumulation. Finally, the presence of high metals concentrations have been observed at the sites of lesions in various neurodegenerative diseases enhancing the apoptotic process by conversion of hydrogen peroxide into hydroxyl radicals by a metal-dependent mechanism. Finally, the true role of protein aggregation in the process of abnormal neuronal apoptosis, which results in degeneration of brain functions and development of the disorder, is still unknown.
Other biological aspects have been associated with the accumulation of misfolded proteins due to various dysfunctions. The proteasome deregulation, because of genetic mutation or oxidative stress, is thought to be also responsible for the accumulation of aggregated proteins within inclusion bodies. Chronic inflammation associated with microglia and mitochondria dysfunctions promote also toxicity and amyloid protein accumulation. Finally, the presence of high metals concentrations have been observed at the sites of lesions in various neurodegenerative diseases enhancing the apoptotic process by conversion of hydrogen peroxide into hydroxyl radicals by a metal-dependent mechanism. Finally, the true role of protein aggregation in the process of abnormal neuronal apoptosis, which results in degeneration of brain functions and development of the disorder, is still unknown.
 Apoptosis is an active process of cell death involved in the development of organisms. Abnormal regulation of apoptotic processes leads to numerous pathologies ranging from neurodegenerative disorders to autoimmunity and cancers. The proteins of the Bcl-2 family are molecular transducers sensitive to internal and external apoptotic signals. Consequently, they play a key role in the regulation of apoptosis. The Bcl-2 family members can be divided in two subgroups: the pro- and anti-apoptotic proteins, acting respectively as activators and inhibitors of apoptosis.
In vivo, the "life-or-death" decision depends on the interactions between various members of the Bcl-2 family through the formation of homo- and hetero-oligomers. The anti-apoptotic members of the family (such as Bcl-2 and Bcl-xL) interact with the pro-apoptotic members (Bax and Bak), hereby inhibiting their activity. Upon induction of apoptosis, this inhibitory interaction is released and the pro-apoptotic proteins can permeabilize the outer mitochondria membrane, leading to the release of cytochrome c within the cytoplasm, and the ongoing of the apoptosis process.
Apoptosis is an active process of cell death involved in the development of organisms. Abnormal regulation of apoptotic processes leads to numerous pathologies ranging from neurodegenerative disorders to autoimmunity and cancers. The proteins of the Bcl-2 family are molecular transducers sensitive to internal and external apoptotic signals. Consequently, they play a key role in the regulation of apoptosis. The Bcl-2 family members can be divided in two subgroups: the pro- and anti-apoptotic proteins, acting respectively as activators and inhibitors of apoptosis.
In vivo, the "life-or-death" decision depends on the interactions between various members of the Bcl-2 family through the formation of homo- and hetero-oligomers. The anti-apoptotic members of the family (such as Bcl-2 and Bcl-xL) interact with the pro-apoptotic members (Bax and Bak), hereby inhibiting their activity. Upon induction of apoptosis, this inhibitory interaction is released and the pro-apoptotic proteins can permeabilize the outer mitochondria membrane, leading to the release of cytochrome c within the cytoplasm, and the ongoing of the apoptosis process.
 Bcl-xL, an anti-apoptotic factor of the Bcl-2 family, is involved in the homeostasis of organelles and is essential for the healthy development of various circulating cells as well as organs like the brain. Besides apoptosis, this protein is also involved in autophagy, cycle cell modulation and neuronal plasticity.
Bcl-xL, an anti-apoptotic factor of the Bcl-2 family, is involved in the homeostasis of organelles and is essential for the healthy development of various circulating cells as well as organs like the brain. Besides apoptosis, this protein is also involved in autophagy, cycle cell modulation and neuronal plasticity.
 In vitro studies have demonstrated that Bcl-xL was able to adopt amyloid conformation and form amyloid fiber by increasing the temperature (Chenal
et al.,
Journal of Molecular Biology, 2012). Depending on the temperature, two types of amyloid fibers of Bcl-xL can be differentiated, the first one, formed at 70°C, that bound Thioflavin-T dye and displayed amyloid structure; and the second one which consisted in oligomers formed at 37°C, faintly binding by Thioflavine T, defining as “native-like” fiber. Moreover, another amyloidogenic Bcl-xL fiber was generated by enzymatic cleavage of the protein, with a high amyloid.
In vitro studies have demonstrated that Bcl-xL was able to adopt amyloid conformation and form amyloid fiber by increasing the temperature (Chenal
et al.,
Journal of Molecular Biology, 2012). Depending on the temperature, two types of amyloid fibers of Bcl-xL can be differentiated, the first one, formed at 70°C, that bound Thioflavin-T dye and displayed amyloid structure; and the second one which consisted in oligomers formed at 37°C, faintly binding by Thioflavine T, defining as “native-like” fiber. Moreover, another amyloidogenic Bcl-xL fiber was generated by enzymatic cleavage of the protein, with a high amyloid.
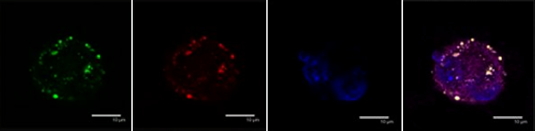
 In cellulo studies had revealed that Bcl-xL can aggregate into amyloid fibers under stress conditions (metals Fe/Zn/Cu, irradiations, hydrogen peroxide). In cells submitted to stress-induced apoptosis, Bcl-xL participates to the formation of inclusion bodies with amyloid features, as revealed by Thioflavin T staining (Figure 2). To prevent apoptosis, Bcl-xL is known to interact with pro-apoptotic members of the Bcl-2 family, but neither Bax nor Bad co-localize with stress-induced inclusion bodies in cells, indicating a specific role of this Bcl-xL transformation. Formation of amyloid deposits might represent a Bcl-xL trap, preventing its anti-apoptotic action, and displacing the balance between anti- and pro-apoptotic towards the death decision, thus participating in the induction or the rate of abnormal apoptosis.
In cellulo studies had revealed that Bcl-xL can aggregate into amyloid fibers under stress conditions (metals Fe/Zn/Cu, irradiations, hydrogen peroxide). In cells submitted to stress-induced apoptosis, Bcl-xL participates to the formation of inclusion bodies with amyloid features, as revealed by Thioflavin T staining (Figure 2). To prevent apoptosis, Bcl-xL is known to interact with pro-apoptotic members of the Bcl-2 family, but neither Bax nor Bad co-localize with stress-induced inclusion bodies in cells, indicating a specific role of this Bcl-xL transformation. Formation of amyloid deposits might represent a Bcl-xL trap, preventing its anti-apoptotic action, and displacing the balance between anti- and pro-apoptotic towards the death decision, thus participating in the induction or the rate of abnormal apoptosis.
 Thus our project is to investigate the relationships between the formation of amyloid deposits and the abnormal apoptosis, which are features common to neurodegenerative disorders. To do so,
in vivo experiments were performed on mouse model of neurodegenerative pathology. Our recent results suggest that Bcl-xL might be important for linking the two events.
Thus our project is to investigate the relationships between the formation of amyloid deposits and the abnormal apoptosis, which are features common to neurodegenerative disorders. To do so,
in vivo experiments were performed on mouse model of neurodegenerative pathology. Our recent results suggest that Bcl-xL might be important for linking the two events.
A
B
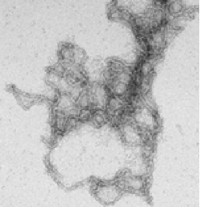
Figure 2:
A)
In cellulo: Cellular immunolocalisation of amyloid aggregate (thioflavin T, green staining) and of Bcl-xL protein (red staining) in apoptotic SH-SY5Y induced by ZnCl2.
B)
In vitro: electronic microscopy of Bcl-xL amyloid fibres. |