C. Gerez, S. Ollagnier de Choudens, M. Pelosse
Maturation of metallic sites such as iron-sulfur clusters (Fe-S), canonical or complex ones (containing additional heteroatoms), remains a very active and competitive field in bioinorganic chemistry. Maturation systems are structurally and functionally complex and more and more diseases
[Reference] are revealed due to defects in Fe-S assembly process, pointing to the importance to study at a molecular level such a process.
The BioCatalysis team was pioneer in the study at a molecular level of Fe-S biogenesis in prokaryotes. In bacteria, two conserved Fe-S assembly systems exist, the housekeeping ISC system and the stress-responsive SUF one. Since the late 1990s, the BioCatalysis team is working intensively on the SUF system, composed by six proteins SufA-SufB-SufC-SufD-SufS-SufE, in collaboration with spectroscopists and microbiologists. The team is also working on the ISC system, composed by seven proteins (IscS-IscU-IscA-HscA-HscB-Fdx and CyaY). Currently, a particular attention is focusing on the (i) structural and functional aspects of the SufBCD and SufBCDSE complexes with their cofactors (Fe-S and FADH
2); (ii) iron entry to these complexes to form the Fe-S cluster; (iii) biochemical and biophysical characterization of ISC mutants allowing to bypass frataxin (CyaY), as a strategy to threat Friedreich ataxia in eukaryotes; (iv) biochemical and biophysical characterization of A-Type proteins (SufA, NfuA, IscA, …both wt and variants) and some electron transfer systems allowing an efficient biosynthesis of valuable compounds in cellulo (NAD, Biotin, bisabolene, antibiotics…).
A deep investigation of the eukaryotic system involved in Fe-S biogenesis is essential to better arrest diseases related to Fe-S assembly defects. We are working on the mammalian mitochondrial ISC machinery (Figure 1). Among the twenty proteins involved in this process, we are focusing on (i) ISCA1/2 proteins and its partners involved in the late step of the process and on frataxin (FXN), a protein involved in the initial stage of Fe-S biogenesis and whose deficiency causes the severe neurodegenerative disease Friedreich Ataxia (FRDA).
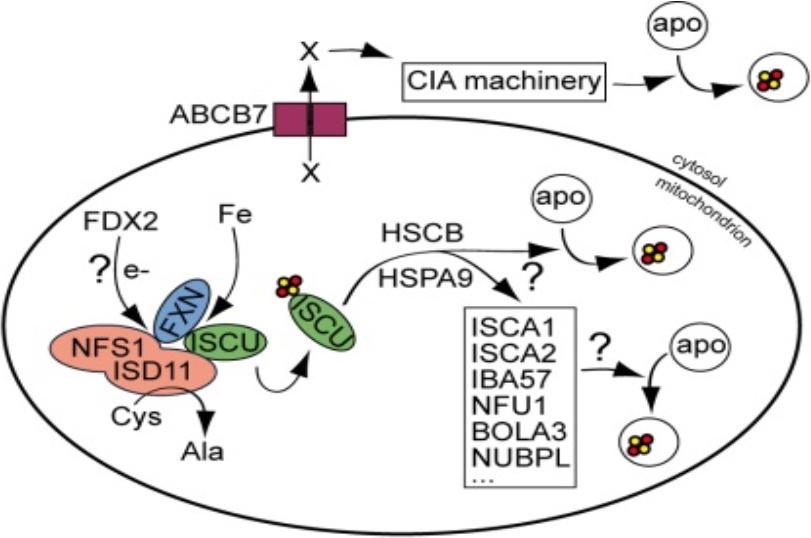
Figure 1: Mitochondrial ISC machinery.
e-: electron, Fe: iron, apo: apoprotein, X:unknown, CIA: cytosolic Fe-S cluster, red and yellow spheres: iron and sulfur atoms respectively.
 Collaborations on the Fe-S biogenesis projects
Collaborations on the Fe-S biogenesis projects F. Barras (Pasteur Institut, Paris); B. Py (LCB, Marseille); H. Puccio (INMG, Lyon); JM. Latour/ G. Blondin (PMB, LCBM, Grenoble); G. Veronesi (MCT, LCBM, Grenoble); J. Pérard (BEE, LCBM, Grenoble); G. Bokinsky (Delf, Netherlands); J.C. Fontecilla-Camps (Métalloprotéines, IBS, Grenoble); S. Cianferani (IPHC, Strasbourg).
 Financial supports
Financial supports National Agency of Research (ANR FRACOL, ANR EraCoBioTech IronPlugNPlay)
 Selected publications on the Fe-S biogenesis projectPy B, Gerez C, Huguenot A, Vidaud C, Fontecave M, de Choudens SO and Barras F
Selected publications on the Fe-S biogenesis projectPy B, Gerez C, Huguenot A, Vidaud C, Fontecave M, de Choudens SO and Barras F
The ErpA/NfuA complex builds an oxidation-resistant Fe-S cluster delivery pathway.
Journal of Biological Chemistry, 2018,
293(20): 7689-7702
Pérard J and Ollagnier de Choudens S Iron–sulfur clusters biogenesis by the SUF machinery: Close to the molecular mechanism understanding.
Journal of Biological Inorganic Chemistry, 2018,
23(4): 581-596
Correction.
Journal of Biological Inorganic Chemistry, 2018,
23(4): 597
Beilschmidt LK, Ollagnier de Choudens S, Fournier M, Sanakis I, Hograindleur MA, Clémancey M, Blondin G, Schmucker S, Eisenmann A, Weiss A, Koebel P, Messaddeq N, Puccio H and Martelli A ISCA1 is essential for mitochondrial Fe
4S
4 biogenesis
in vivo.
Nature Communications, 2017,
8: Article number 15124
Blanc B, Clémancey M, Latour JM, Fontecave M and Ollagnier de Choudens S Molecular investigation of Iron-Sulfur cluster assembly scaffolds under stress.
Biochemistry, 2014,
53(50): 7867-77869
Colin F, Martelli A, Clemancey M, Latour JM, Gambarelli S, Zeppieri L, Birck C, Page A, Puccio H and Ollagnier de Choudens S Mammalian frataxin controls sulfur production and iron entry during
de novo Fe
4S
4 cluster assembly.
Journal of the American Chemical Society, 2013,
135(2): 733-740
Tsaousis AD, Ollagnier de Choudens S, Gentekaki E, Long S, Gaston D, Stechmann A, Vinella D, Py B, Fontecave M, Barras F, Lukes J and Roger AJ Evolution of Fe/S cluster biogenesis in the anaerobic parasite
Blastocystis.
Proceedings of the National Academy of Sciences, 2012,
109(26): 10426-10431
Wollers S, Layer G, Garcia-Serres R, Signor L, Clemancey M, Latour JM, Fontecave M and Ollagnier de Choudens S Iron-sulfur (Fe-S) cluster assembly: The SufBCD complex is a new type of Fe-S scaffold with a flavin redox cofactor.
Journal of Biological Chemistry, 2010,
285(30): 23331-23341
Gupta V, Sendra M, Naik SG, Chahal HK, Huynh BH, Outten FW, Fontecave M and Ollagnier de Choudens S Native
Escherichia coli SufA, coexpressed with SufBCDSE, purifies as a [2Fe-2S] protein and acts as an Fe-S transporter to Fe-S target enzymes.
Journal of the American Chemical Society, 2009,
131(17): 6149-6153
Loiseau L, Gerez C, Bekker M, Ollagnier-de Choudens S, Py B, Sanakis Y, Teixeira de Mattos J, Fontecave M and Barras F ErpA, an iron sulfur (Fe S) protein of the A-type essential for respiratory metabolism in
Escherichia coli.
Proceedings of the National Academy of Sciences USA, 2007,
104(34): 13626-13631
Ranquet C, Ollagnier de Choudens S, Loiseau L, Barras F and Fontecave M Cobalt stress in
Escherichia coli: The effect on the iron-sulfur proteins.
Journal of Biological Chemistry, 2007,
282(42): 30442-30451
Layer G, Ollagnier-de Choudens S, Sanakis Y and Fontecave M Iron-sulfur cluster biosynthesis: characterization of
Escherichia coli CYaY as an iron donor for the assembly of [2Fe-2S] clusters in the scaffold IscU.
Journal of Biological Chemistry, 2006,
281(24): 16256-63
Loiseau L, Ollagnier de Choudens S, Fontecave M and Barras F Analysis of the
Escherichia coli CsdA-CsdE heterodimeric cysteine desulfurase, a new [Fe-S] biogenesis system.
Journal of Biological Chemistry, 2005,
280: 26760-26769
Loiseau L, Ollagnier de Choudens S, Nachin L, Fontecave M and Barras F Biogenesis of Fe-S cluster by the bacterial Suf system: SufS and SufE form a new type of cysteine desulfurase.
Journal of Biological Chemistry, 2003,
278: 28352-28359
Ollagnier de Choudens S, Sanakis Y, Loiseau L, Nachin L, Barras F and Fontecave M SufA from
Erwinia chrysanthemi: Characterization of a scaffold protein required for iron-sulfur cluster assembly.
Journal of Biological Chemistry, 2003,
278: 17993-18001
Ollagnier de Choudens S, Mattioli T, Takahashi Y and Fontecave M Iron-sulfur cluster assembly: Characterization of IscA and evidence for a specific and functional complex with ferredoxin.
Journal of Biological Chemistry, 2001,
276: 22604-22607
 Books
Puccio H and Ollagnier de Choudens S
Books
Puccio H and Ollagnier de Choudens S
Iron-sulfur cluster assembly in bacteria and eukarya using the ISC biosynthesis machinery Encyclopedia of Inorganic and Bioinorganic Chemistry, 2017 In ‘
Metalloprotein Active Site Assembly’, edited by Michael K. Johnson and Robert A. Scott. Chichester, UK: John Wiley & Sons, Ltd, pp. 1-19.
Barras F and Ollagnier de Choudens S
Genetic, biochemical and biophysical methods for studying Fe-S proteins and their assembly Meth. Enzymol. 2017, Vol 595, Edited by Sheila S. David, pp. 1-32.