Principal Investigator: Giulia Veronesi
At the cutting edge of nanotechnology, metallic nanoparticles (NP) find applications today in the biomedical field, for imaging, sensing, and therapy. Moreover, metallic NP are massively used in consumer products like food packaging or cosmetics, as antibacterial agents or sunscreens, for instance.
On the one hand, it is crucial to assess the stability of engineered nanomaterials for biomedical applications in in vitro
biological models, in order to contribute to the design of biocompatible functional nanomaterials with intact optical properties and ideally no toxicity.
On the other hand, we are concerned about the release of NP from consumer products. Therefore, it is of paramount importance to address the question of the fate of these materials by exploring their transformations in human cells and in the environment.
Synchrotron techniques as X-ray Fluorescence (XRF) nano-imaging and X-ray Absorption Spectroscopy (XAS) are unique experimental techniques that can reveal the fate of metallic NP in cells and tissues. XRF nano-imaging provides the sub-cellular distribution of native and exogenous elements, allowing for the visualization of the trafficking pathways of the metals in a single cell, their distribution, and the target cell compartments.
XAS interrogates a selected metal and provides information about its average speciation, coordination, and oxidation state. Therefore, it is an invaluable tool to disclose the physicochemical transformations of metallic NP in biological media.
Thanks to a long-lasting collaboration with the European Synchrotron Radiation Facility (ESRF), and to the continuous interaction with experts in cell and molecular biology at the LCBM, we conducted a large number of studies with the common aim to disclose the fate of metallic NP in biological systems with the help of synchrotron techniques.
 Silver nanoparticles in hepatic cells: Physicochemical transformations and toxicity mechanisms
Main collaborators
Silver nanoparticles in hepatic cells: Physicochemical transformations and toxicity mechanisms
Main collaborators: LCBM/Met&Or team, CEA-Grenoble. ESRF beamlines BM30 and BM16B, Grenoble. Institute of Structural Biology (IBS), Grenoble. SyMMEs/CIBEST, CEA-Grenoble.
Nanosilver is widely used for its antibacterial properties in consumer products and medical devices. Its toxicity and eco-toxicity have been a matter of concern over the last decade, considering the massive release of silver in the environment and the direct exposure of humans. The main exposure routes are the gastro-intestinal and the parenteral ones, both leading to the accumulation of different forms of Ag, nanoparticles (NP)
vs ionic, in the liver.
We investigated the fate of silver nanoparticles
in vitro in hepatic cells, including classic 2D cultures and 3D pseudo-livers. By making use of synchrotron techniques as XAS and XRF imaging, we highlighted the transformations of the NP
in cellulo, and correlated them to the observed cytotoxicity and gene expression in exposed cells.
We developed a multimodal single cell imaging protocol, which allows for the simultaneous visualization of the cellular compartments, of silver NP, and of the soluble fraction of Ag(I) released from the NP surface (
Figure1C, D)
[1]. In parallel studies, we investigated the molecular complexes formed by Ag(I) with organothiols and metalloproteins (
Figure 1A), and the grafting of thiolate biomolecules to the silver NP surface
[2, 3].
The direct observation of silver trafficking in cells exposed to AgNP and the fine investigation of the Ag species formed
in cellulo (
Figure1B) allowed us to gain insight into mechanisms of toxicity as the interference with the cellular metal homeostasis and the impairment of the nuclear receptors’ activity
[4, 5].
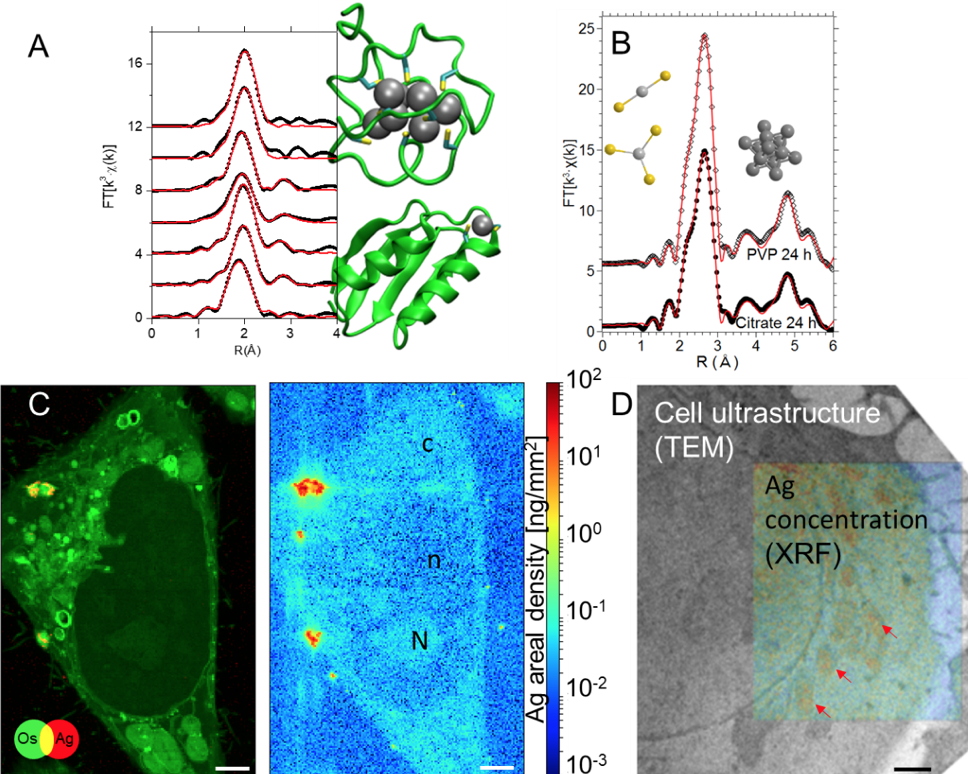 Figure 1
Figure 1. (A) Ag K-edge XAS study of Ag(I) binding in biological Cu(I) sites
[2].
(B) Ag K-edge XAS spectra of hepatocytes exposed to Ag NP. The
ab initio fits (red curves) to the experimental spectra reveal the simultaneous presence of silver in nanoparticles and in Ag(I)-organothiol compounds
[4].
(C) Os (added as a fixative) and Ag distribution in a single hepatocyte exposed to AgNP, extracted from XRF hyperspectral images, and quantitative silver distribution in the same map area. Subcellular compartments as nucleus (n), nucleoli (N), and cytoplasm (c) can be distinguished. Scale bars = 2 μm
[1].
(D) Example of multimodal imaging used to highlight the presence of Ag in mitochondria (red arrows) in hepatocytes exposed to AgNP.
Scale bar = 1 μm
[1]
 Related publications
Related publications:
[1] V. Tardillo Suárez, B. Gallet, M. Chevallet, P.-H. Jouneau, R. Tucoulou, G. Veronesi, A. Deniaud. Correlative transmission electron microscopy and high-resolution hard X-ray fluorescence microscopy of cell sections to measure trace element concentrations at the organelle level.
Journal of Structural Biology (2021),
213: 107766.
[2] G. Veronesi, T. Gallon, A. Deniaud, B. Boff, C. Gateau, C. Lebrun, C. Vidaud, F. Rollin-Genetet, M. Carrière, I. Kieffer, E. Mintz, P. Delangle, I. Michaud-Soret. XAS investigation of silver(I) coordination in copper(I) biological binding sites.
Inorg. Chem. (2015),
54 : 11688-11696.
[3] M. Marchioni, C. Battocchio, Y. Joly, C. Gateau, S. Nappini, I. Pis, P. Delangle, I. Michaud-Soret, A. Deniaud, G. Veronesi. Thiolate-Capped Silver Nanoparticles: Discerning Direct Grafting from Sulfidation at the Metal-Ligand Interface by Interrogating the Sulfur Atom.
J. Phys. Chem. C (2020),
124: 13467–13478.
[4] G. Veronesi, A. Deniaud, T. Gallon, P.-H. Jouneau, J. Villanova, P. Delangle, M. Carrière, I.Kieffer, P. Charbonnier, E. Mintz and I. Michaud-Soret. Visualization, quantification and coordination of Ag
+ ions released from silver nanoparticles in hepatocytes.
Nanoscale (2016),
8: 17012-17021
[5] V. Tardillo Suárez, E. Karepina, M. Chevallet, B. Gallet, C. Cottet-Rousselle, P. Charbonnier, C. Moriscot, I. Michaud-Soret, W. Bal, A. Fuchs, R. Tucoulou, P.-H. Jouneau, G. Veronesi, A. Deniaud. Nuclear translocation of silver ions and hepatocyte nuclear receptor impairment upon exposure to silver nanoparticles.
Environ. Sci.: Nano (2020),
7: 1373-1387.
 In vivo/in cellulo stability of Quantum Dots (QD) for biomedical applications
Main collaborators
In vivo/in cellulo stability of Quantum Dots (QD) for biomedical applications
Main collaborators: SyMMES/STEP, CEA-Grenoble. LCBM/Met&Or team, CEA-Grenoble. ESRF beamlines ID21 and BM23, Grenoble. ISASI/Nanobiobolecular group, CNRS Pozzuoli (IT).
Thanks to their outstanding optical properties and to the large opportunities offered by surface functionalization, Quantum Dots (QD,
i.e. semiconductor nanocrystals) are prime candidates for theranostic applications, which aim at combining diagnosis, targeting, and therapy agents on a single nanoplatform. First generation QD were based on CdS and CdSe, intrinsically toxic because of the possible release of Cd from their core, hence unsuitable for biomedical applications. Promising candidates for such applications are InP-based QD, since they are considered as possibly non-toxic, and their emission can be tuned in the entire visible spectral range. However, gaps in the knowledge of their intracellular fate, persistence, and excretion from the targeted cell or organism still exist, precluding clinical applications.
We probed the stability of two InPZnS core/shell formulations of QD in the model organism
Hydra vulgaris. We made use of cryogenic XRF imaging to visualize the entry and the biodistribution of the QD in the tissue in nearly native conditions. With In L-edge XAS, we assessed the transformations of the QD in the animal. We observed a degradation of the InPZnS core, followed by the formation of In-carboxylate species (
Figure 2). Such transformations are observed after 24 hours
in vivo, whereas the colloidal suspensions of QD were stable after the same time
in vitro at acidic pH. The degradation of the core of the QD originates a loss of the optical properties
in vivo [1]. XRF imaging and XAS provided insights into the biotransformation of QD in a model organism, and can help assess the performance of new core/shell formulations with improved stability.
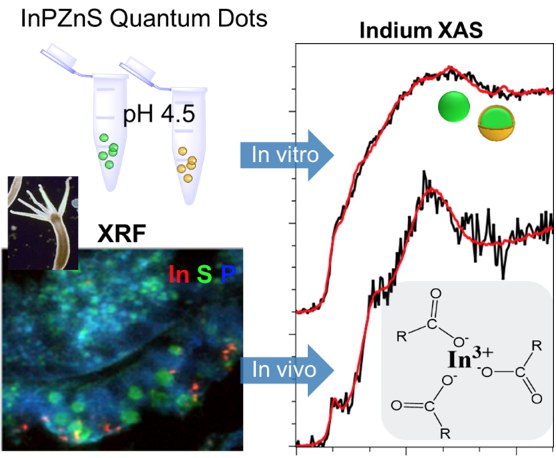 Figure 2
Figure 2. XRF imaging and In L-edge XAS reveal the transformation of InPZnS QD in the model organism
Hydra vulgaris. Both in the presence and absence of a Zn(Se,S) gradient shell, a degradation of the InPZnS core and a formation of In-carboxylate species is observed after 24 hours
in vivo [1].
 Related publication
Related publication:
[1] G. Veronesi, M. Moros, H. A. Castillo-Michel, L. Mattera, G. Onorato, K. D. Wegner, W. Li Ling, P. Reiss, C. Tortiglione.
In vivo biotransformations of indium phosphide quantum dots revealed by X-ray micro-spectroscopy.
ACS Appl. Mater. Interfaces (2019),
11: 35630-35640.