Metal transporters: Mechanism and selectivity
Published on 3 October 2020
Body text 1
Patrice Catty, CEA scientist
Elisabeth Mintz, CEA scientist
Roger Miras, CEA senior technician
 Research topics
Research topics
For several years, we are studying P-type ATPases, membrane proteins carrying ions from one side to the other side of a lipid bilayer, against their concentration gradient and for this active transport the energy provided by the hydrolysis of ATP (Figure 1).
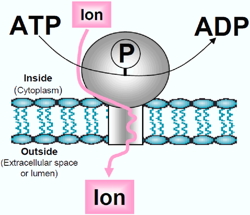 | |
Figure 1: Within the P-type ATPase, ion transport, in the opposite direction to the concentration gradient, is coupled to the hydrolysis of a molecule of ATP. The P-type ATPase is transiently phosphorylated during transport. |
A classification of P-type ATPases has been proposed by Palmgren and Axelsen (1998) that accounts for the ion selectivity of these carriers. Specialized in the transport of the so-called “heavy metals” P-type ATPases of the PIB family carry Cu(I), Ag(I), Cd(II), Co(II), Pb(II) or Zn (II) (Figure 2). They participate with other carriers to remain in the cell of a non-toxic concentration of these metals.
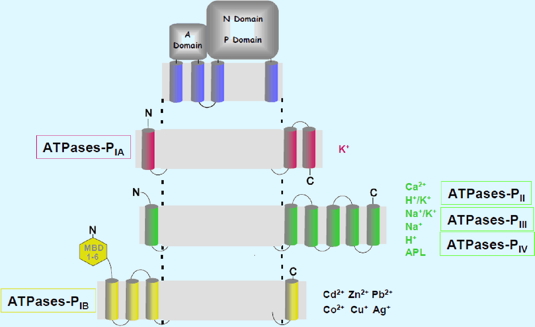
Figure 2: Structural organization of P-type ATPases.
P-type ATPases have a catalytic domain consisting of domains A (actuator), P (phosphorylation) and N (nucleotide binding). They are embedded in the membrane by a domain of variable size (6 to 12 transmembrane helices). Unlike the other P-type ATPases, PIB-type ATPases have 1-6 N-terminal metal binding (MBD).
Our study models:
• CadA, ATPase-Cd(II) of Listeria monocytogenes, regarded as the model PIB-type ATPase
• CoaT the ATPase-Co(II) of Synechocystis PCC6803, essential to the survival of the bacteria in an environment contaminated by cobalt.
• Ccc2, ATPase-Cu(I) of Saccharomyces cerevisiae, model of human ATPases-Cu(I).
• In collaboration with the laboratory PCV/iRTSV (Daphné Seigneurin-Berny), we also work on PAA1, an ATPase-Cu(I) of the chloroplast envelope but also on two other PIB-type ATPases of the chloroplast, namely HMA1 and PAA2.
Few words on metals we are interested in
Whatever their effects on the living, Cd(II), Co(II) and Cu(I) have common chemical properties that make them capable of covalent bonds with sulfur atoms of cysteine and methionine and nitrogen atoms of histidine.
Cadmium is one of the major environmental pollutants of anthropogenic origin. At the cellular level, cadmium is known to cause DNA damage and inhibit certain repair systems of genetic material. Its toxicity is also manifested by a disturbance of homeostasis of certain physiological metals such as calcium and by inhibiting the activity of certain enzymes. Finally, cadmium can indirectly generate oxidative stress within the cell. The response of the living to the cytotoxic effects of cadmium involves three complementary types of cellular responses: complexation, sequestration and efflux of the metal. Usually the complexation involves small cytoplasmic proteins like glutathione or phytochelatin which by their chemical properties bind cadmium to reduce its chemical reactivity. Sequestration consists of a metal of isolation in a given compartment of the cell. The efflux of cadmium is in turn essentially ensured by P-type ATPases able to expel the Cd(II) to the surroundings allowing the survival of microorganisms in polluted environments.
Known to be associated with vitamin B12, cobalt is also the cofactor of certain bacterial and human enzymes. It can be toxic in humans where it is classified as potentially carcinogenic. Considered as trace element in natural environments, cobalt can concentrate almost a thousand times in polluted ponds or rivers. It is tempting to link the proliferation of cyanobacteria in these polluted ponds or rivers to the presence of specific and efficient cobalt transporters, among them P-type ATPases. Cobalt transporting P-type ATPases have not been yet functionally studied are found in only a small number of bacterial species including cyanobacteria, M. tuberculosis and certain pathogens responsible for nosocomial infections. These ATPases could be essential for their survival in a hostile environment (remember that many metals are known and used in hospitals for their bactericidal activity). The study Coat from Synechocystis PCC6803 is conducted to obtain a sufficiently precise knowledge of the transport mechanism of Co(II) and to find a specific inhibitor of this type of transporter.
Copper is an essential metal in the cell life since the redox properties of the couple Cu(I)/Cu(II) make it a cofactor in many enzymatic reactions in all living organisms. In mammals, there are two Cu(I) transporting ATPases and two severe genetic diseases associated with mutations in their genes, Menkes disease and Wilson disease. Yeast possesses one Cu(I)-ATPase that serves as a model for studying human ATPases. In eukaryotes, the Cu(I)-ATPase is located at the Golgi membrane where it supplies certain proteins of the secretory pathway with copper. In the event of flooding of the cell by the copper, Cu(I)-ATPase containing vesicles migrate to the plasma membrane moving the transporters nearby the cell surface where they can actively expel copper excess. It is through this migration of vesicles to the plasma membrane that occur uptake of copper from the gut and detoxification of copper in the liver.
 Techniques
Molecular biology - heterologous expression in various systems (bacteria, yeast, insect cells) - cell culture - yeast genetic - protein purification - biochemistry - absorption spectroscopy - intrinsic fluorescence - circular dichroism - light scattering - immunofluorescence - quantitative PCR.
Techniques
Molecular biology - heterologous expression in various systems (bacteria, yeast, insect cells) - cell culture - yeast genetic - protein purification - biochemistry - absorption spectroscopy - intrinsic fluorescence - circular dichroism - light scattering - immunofluorescence - quantitative PCR.
Top page