 Leader
Leader
| Dr. Geneviève Blondin
Laboratoire Chimie et Biologie des Métaux
CEA-Grenoble
17 avenue des Martyrs
38 054 Grenoble Cedex
email: Genevieve.Blondin@cea.fr
Phone: 04 38 78 38 47
Fax: 04 38 78 54 65 |
Biophysical methods are invaluable to characterize metalloprotein active sites and elucidate their activity. Their direct study is supported by a thorough investigation of model complexes that provide spectroscopic signatures of key species and intermediates, what, in turn, paves the way to mechanistic analyses. The main activities of the group in this domain are focused on the use of Mössbauer spectroscopy to study iron proteins and model complexes. They are focused on three domains: iron accumulation and storage, iron-sulfur biosynthesis and degradation and dioxygen activation by iron enzymes and models.
| Dr. Ricardo Garcia
Laboratoire Chimie et Biologie des Métaux
CEA-Grenoble
17 avenue des Martyrs
38 054 Grenoble Cedex
email: Ricardo.Garcia@cea.fr
Phone: 04 38 78 62 06
Fax: 04 38 78 54 65 |
| Dr. Martin Clémancey
Laboratoire Chimie et Biologie des Métaux
CEA-Grenoble
17 avenue des Martyrs
38 054 Grenoble Cedex
email: martin.clemancey@cea.fr
Phone: 04 38 78 02 06
Fax: 04 38 78 54 65 |

 Permanent staff involved in the project
Permanent staff involved in the project
Geneviève Blondin
Martin Clémancey
Richard Garcia
Jean-Marc Latour

 Non permanent staff involved
Non permanent staff involved
| Velavan Kathirvelu | Post-doc | 04/2012 - 03/2013 |
| Alexandra Sève | M2 | 01/2013 - 06/2013 |
| François Dorandeu | IUT | 04/2012 - 06/2012 |
 Présentation
Présentation
 Mössbauer spectroscopy
Mössbauer spectroscopy
For an introduction to Mössbauer spectroscopy see Prof. P. Gütlich homepage.
 Mössbauer equipment
Mössbauer equipment
Oxford Instrument cryomagnet operating at 1.4 - 300 K up to 8 T applied either parallel or perpendicular to the &Mac179;-ray
Janis
Home-made cryostat
 A recent example of Mössbauer study
A recent example of Mössbauer study
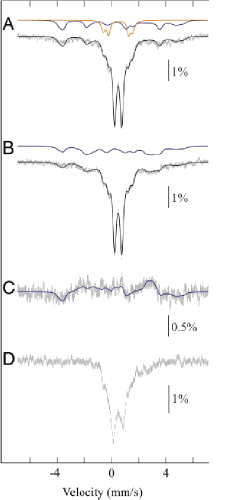 Mössbauer spectra of as-isolated enzyme MiaE, a diiron oxygenase involved in the post-transcriptional modification of tRNA
Mössbauer spectra of as-isolated enzyme MiaE, a diiron oxygenase involved in the post-transcriptional modification of tRNA
Mössbauer spectra of MiaE2H (1.9 mM) in 100 mM Tris_HCl (pH 8) containing 30 mM NaCl and 5 % glycerol. Experimental conditions: spectra were recorded at 4.2K in a magnetic field of 60mT applied parallel to the beam(A), or 22 mT applied perpendicular to the beam (B), or at 77K and zero applied field (D). Spectrum C is obtained by subtraction (A - B). The solid black lines are spin-Hamiltonian simulations, generated by using the parameters described below. The solid colored lines are contributions from the oxodiferric clusters (orange) and the mixed-valence [FeII-FeIII] clusters (blue). Contribution from the majority diferric species (central doublet) is not shown.
Interpretation
These spectra reveal the presence of three different forms of the dinuclear site:
(i) an EPR-silent hydroxo-bridged FeIII-OH-FeIII center, with characteristic Mössbauer spectroscopic properties (δ=0.49 mm/s, Δ
EQ=0.51 mm/s);
(ii) an EPR-silent oxo-bridged FeIII-O-FeIII center, characterized by oxo-to-iron charge transfer bands in the UV-visible spectrum and by large quadrupole splittings of the corresponding doublets in the Mössbauer spectrum (δ
=0.52 mm/s, Δ
EQ=1.49 mm/s; δ
=0.48 mm/s, Δ
EQ=2.16 mm/s);
(iii) a hydroxo-bridged FeII-OH-FeIII mixed-valent center, remarkably stable and displaying a characteristic rhombic signal at g values &Mac178;2 in the EPR spectrum as well as a broad magnetic signal in the Mössbauer spectrum, in agreement with a S = 1/2 ground state.
See
Mathevon C, Pierrel F, Oddou JL, Garcia-Serres R, Blondin G, Latour JM, Menage S, Gambarelli S, Fontecave M and Atta M
tRNA-modifying MiaE protein from Salmonella typhimurium is a nonheme diiron monooxygenase.
Proceedings of the National Academy of Sciences USA, 2007, 104(33): 13295-13300
 EPR spectroscopy
EPR spectroscopy
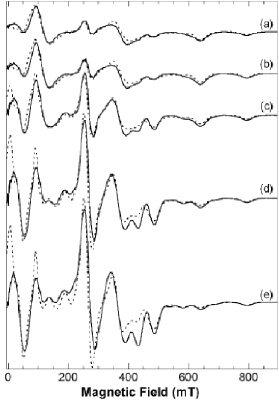
EPR spectroscopy is a very powerful method to analyze metalloprotein active sites possessing unpaired spins and derive molecular information. We use it to characterize the active sites of manganese and iron proteins. A detailed methodology to analyze the temperature and magnetic field dependence of EPR spectra of binuclear manganese(II) species has been recently proposed.
EPR spectra of a binuclear manganese complex
Experimental (continuous line) and simulated (dotted line) X-band spectra at several temperatures: (a) 4.3 K, (b) 6.8 K, (c) 9.2 K, (d) 13.8 K, (e) 18.0 K. All spectra were scaled to the recording conditions of spectrum (a). See text for the simulated spectra.
See
Dubois L, Xiang DF, Tan XS, Pécaut J, Jones P, Baudron S, Le Pape L, Baffert C, Chardon-Noblat S, Collomb MN, Deronzier A and Latour JM
Binuclear manganese compounds of potential biological significance. 1. Syntheses and structural, magnetic and electrochemical properties of dimanganese(II) and -(II,III) complexes of a bridging unsymmetrical phenolate ligand.
Inorganic Chemistry, 2003, 42(3): 750-760
Blanchard S, Blain G, Rivière E, Nierlich M and Blondin G
Temperature dependence of X- and Q-band EPR spectra of the dinuclear manganese(II) complex [(NO2Bpmp)Mn2(μ-OAc)2]+: Determination of the exchange constant and of the spin parameters for the S=1, 2, and 3 spin states.
Chemistry, a European Journal, 2003, 9(17): 4260-4268