Metal homeostasis, metalloregulatory proteins and their involvement in bacterial virulence
Published on 12 April 2021
Body text 1
Isabelle Michaud-Soret, DR2 CNRS scientist
Although many metals are essential to life, they are toxic at high concentrations and their intracellular concentration is strictly controlled by metalloregulatory proteins that modulate the expression of the genes involved in the transport, storage and metabolism of metals.
In the team we study in particular two regulatory metalloproteins FUR and NikR from Escherichia coli (Ec) and Helicobacter pylori (Hp, a human pathogen associated with cancer and stomach ulcers). In order to understand their mechanism of action and see if they can be good antibacterial targets.
Both of these transcriptional regulators are not only involved in metal homeostasis but are global regulators controlling a large variety of genes. Moreover, in H. pylori, NikR and FUR are part of a fascinating cross-regulatory network that is essential for virulence. The link between metal homeostasis and virulence has been also proved in many cases.
The molecular mechanisms of activation (by the metal ion binding to the protein and interaction of the protein-metal complex with DNA) were studied by multidisciplinary approaches combining molecular biology, biochemistry and biophysical studies (spectroscopic and structural) to correlate biological observations to molecular behavior.
These studies have allowed us to obtain mechanistic information on FUR and NikR which are summarized below:
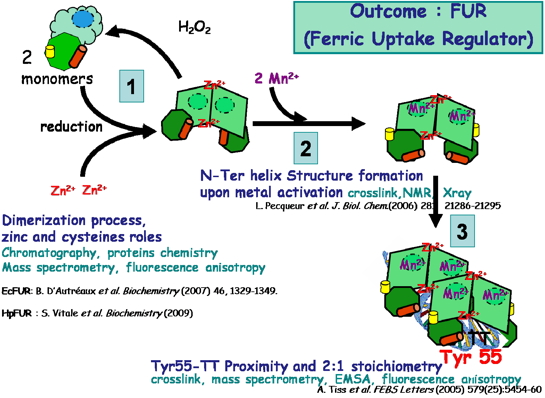
• The structural characterization (NMR and X-rays), the role of zinc and cysteine in the dimerization process of Escherichia coli FUR (EcFUR) [Pecqueur et al., 2006, D'Autreaux et al., 2007] HpFUR [Vitale et al., 2009; Dian et al., 2011] (Coll. IBS and ESRF) and modeling data on the binding site of regulatory ferrous ion [Ahmad et al., 2009] (Coll. R Ahmad, Norway) have was obtained.
• The importance of the close proximity of tyrosine 55 (in the recognition helix for DNA binding) with a pair of highly conserved thymine in FUR boxes found in the promoter regions of genes controlled by FUR has been demonstrated by FUR- DNA crosslink studies followed by mass spectrometry. This tyrosine, essential for the protein / DNA interaction is conserved in all FUR and FUR-like proteins [Tiss et al., 2005] (Coll. IBS).
FUR of Helicobacter pylori is involved in the response to acid stress and is important for gastric colonization in animal models.
We recently obtained the crystal structure of a functionally active mutant HpFUR (HpFUR2M; C78S-C150S) bound to zinc (II) [Dian et al., 2011]. This structure shows an N-terminal extension and a single structured C-terminal helix unusual. The structure also shows three types of metal sites: S1 site previously characterized structural ZnS4 [Vitale et al., 2009] and zinc at two sites identified in other proteins FUR. These two sites influence the binding affinity to the DNA of HpFUR, but only one of them is absolutely required for DNA binding and is responsible for the conformational change induced by the metal binding site The other is a secondary site.
• The importance of the binding of Ni (II) and EcNikR HpNikR function of pH and its role in the homeostatic control of nickel in response to acidity have been studied [Fauquant et al., 2006] and we demonstrated sub-micromolar affinity of EcNikR for Ni (II) [Diederix et al., 2008] (Coll. MA Mandrand, INSA Lyon, L Terradot, ESRF, H de Reuse, Institut Pasteur). The role of a secondary site metal binding has been elucidated in HpNikR using structural characterization, spectroscopic and functional [Bahlawane et al., 2010; Muller et al., 2011]. This secondary site is proposed to modulate the binding HpNikR on certain promoters in response to metal ions other than nickel. This result is an interesting demonstration of interference between the homeostasis of metals.
s.jpg)
A) a new control mechanism of HpNikR dependent on the metal
B) structural characterization and
C) spectroscopic properties: absorbance at 305nm followed as a function of the Ni(II)/protein ratio
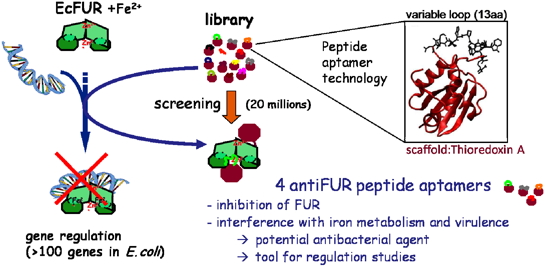
In addition to test FUR as new antibacterial target, we used the peptide aptamers technology (combinatorial protein consisting of a platform of constant and variable peptide loop). Four anti-FUR peptide aptamers were selected for their ability to interact with EcFUR by screening a library of 20 million molecules using a two-hybrid assay in yeast). These four molecules specifically inhibit FUR in vivo and in vitro and affect the virulence of a strain of E. coli pathogen in an infection model of Drosophila [Abed et al., 2007]. These molecules were leads to the discovery of future antibacterial agents. Furthermore, this study has highlighted the interest of peptide aptamers as tools to dissect a complex network of regulations (Coll. Colas P: ENS Lyon and Aptanomics startups and S Crouzy, LCBM).
 Techniques
Techniques
biochimie - purification de protéines - expression hétérologue dans des bactéries - microbiologie et biologie moléculaire - spectroscopies d'absorption - fluorescence- dichroïsme circulaire - diffusion de lumière - double hybride - PCR quantitative-interaction ADN-Protéine (EMSA) - dosage de métaux
Top page