The active site of many enzymes is deeply buried inside their fold, which houses a network of tunnels allowing the transport of substrates between the surface and the active site of the enzymes. The architecture of these tunnels strongly influences the functioning of these enzymes. To understand its mechanism, it is essential to decipher the structure/function relationship of the tunnel systems. The narrowness, the sinuosity and the transient openings of these tunnels make their study difficult in crystallographic structures.
Hydrogenases reversibly catalyze the reduction of 2 protons into molecular hydrogen. They allow the production of hydrogen in specific microorganisms and can serve as catalysts in biofuel cells. In these enzymes, hydrogen diffuses toward or from the active site
via hydrophobic tunnels. Most hydrogenases operate under anaerobic conditions since these enzymes are in general inactivated by oxygen.
Ralstonia eutropha (ReMBH) hydrogenase- [NiFe] is O2-tolerant, and is therefore a target of interest for biotechnological applications.
A collaboration between the researchers of the Biocatalysis team of the Chemistry and Biology of Metals laboratory, the IBS, the ESRF and the Charité-Universitätsmedizin in Berlin allowed to study the relationship between the tunnel architecture of ReMBH and its tolerance to oxygen. For this study, they developed a method for labeling oxygen tunnels in crystallized enzymes. It consists of introducing oxygen or krypton in the enzyme crystals under pressure, the gas populates the binding sites for oxygen and hydrogen, which by contrast to with the protein reveals unambiguously the structure of the tunnels.
The researchers were thus able to map the ReMBH tunnels (Figure A), which allowed them to demonstrate that oxygen and hydrogen are sharing the same routes (Figure B). A structural comparison between different hydrogenases has revealed that O2-tolerant enzymes contain a network of channels with fewer branches than their sensitive counterparts. This characteristic probably allows for a better selectivity of hydrogen. Molecular Dynamics simulations demonstrated that the ReMBH tunnel system has a discriminating mechanism that favors the direct diffusion of molecular hydrogen between the solvent and the active site, but which preferentially redirects molecular oxygen toward secondary branches and thus partially protects the enzyme from inactivation.
Understanding the tunnel architecture (Figure C) is essential to improve the properties of the ReMBH hydrogenase. By modifying the openings size and hydrophobicity of tunnels, it is possible to increase the accessibility of hydrogen and the tolerance of the enzyme to oxygen.
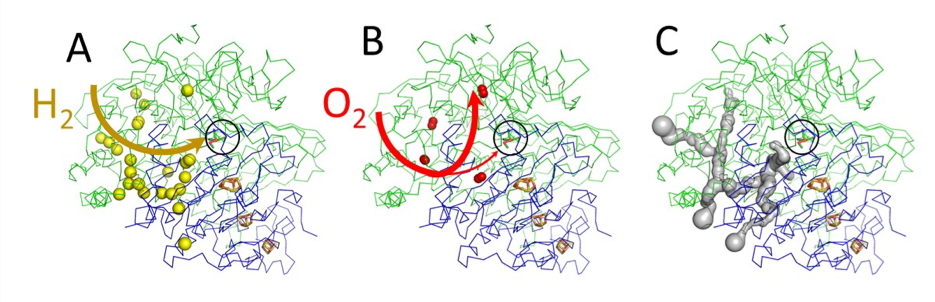
A - Krypton labeling of ReMBH hydrogenase, access of hydrogen to the active site.
B – Oxygen path.
C - Modeling of the tunnel network