An artificial metalloenzyme (ArM) is a hybrid construction resulting of the combination of a synthetic inorganic complex and a biological macromolecule (DNA or protein). Together they form an eco-compatible catalyst that works under mild conditions in accordance with the principles of green chemistry. However, it would be interesting to improve the properties of these ArMs in order to widen their range of substrates (limited in particular by the solubility in aqueous phase), to increase the number of catalytic cycles (limited by the stability of the proteins in the oxidizing medium) and to widen the diversity of the catalyzed reactions (access to combinations of catalytic processes). An important step in this direction has been taken by proposing these catalysts in solid form in order to achieve heterogeneous catalysis (liquid-solid).
It is in this context that researchers at our laboratory are designing ArMs in order to propose new methods of sustainable catalysis for oxidation reactions. They have thus developed several remarkable systems based in particular on the NikA protein to which various synthetic inorganic complexes of iron, manganese or ruthenium have been anchored. This enzyme, responsible for the transport of nickel in bacteria, can be used as a support for the inorganic catalysts designed by the researchers. The researchers have thus developed an original version of an ArM in which NikA crystals have been stabilized by cross-linking using the Cross-Linked Enzyme Crystals or CLEC technique. This technology improves the stability and the number of catalyst recycles while making it possible to extend the reaction conditions used (solvents, pH, temperatures).
After defining the cross-linking conditions of the ArM crystals and ensuring that they were stable in water-organic solvent mixtures (from 4 to 70°C and in the presence of oxidants), their catalytic properties could be evaluated. The researchers thus showed that these heterogeneous catalysts are more efficient than their soluble counterparts for the sulfoxidation of thioglycolamide derivatives. Indeed, the products of this oxidation reaction could be obtained with an efficiency multiplied by 8 and in the presence of very small quantities of catalyst (0.1%). Moreover, even if limited to four, the number of recycles shows that these systems are effective under harsh conditions since the oxidant used, sodium hypochlorite, is very aggressive.
The great originality of this solid hybrid catalyst is to allow the stabilization of the artificial catalytic site within the protein crystal, this by working in an aqueous medium, in the presence of small quantities of catalyst, itself being recycled. The design of artificial crystalline enzymes thus represents a promising alternative to soluble enzymes or supported enzymes for the future of synthetic biology.
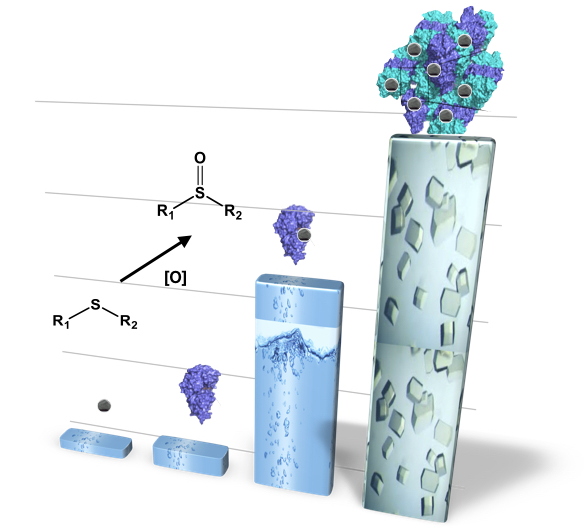 A CLEC-ArM crystal consists of NikA-FeL3 hybrids (FeL3 being a complex of iron with a ligand coordinating the iron by two amine atoms and two pyridines) cross-linked with glutaraldehyde. The stability of the crystals in a water-acetonitrile-1-1 solvent solution makes it possible to use many lipophilic substrates and to test different oxidation reactions, in this case sulfoxidation.
A CLEC-ArM crystal consists of NikA-FeL3 hybrids (FeL3 being a complex of iron with a ligand coordinating the iron by two amine atoms and two pyridines) cross-linked with glutaraldehyde. The stability of the crystals in a water-acetonitrile-1-1 solvent solution makes it possible to use many lipophilic substrates and to test different oxidation reactions, in this case sulfoxidation.
This work combines synthesis, catalysis and crystallographic study of proteins. It is the result of a collaboration between two teams of the Chemistry and Biology of Metals laboratory: the Bioinspired Chemistry and Environment team and the BioCatalysis team.
This work was financed by the ANR CrystalBall (financing of a fixed-term contract and a post-doc) and by the Labex ARCANE which financed the thesis of Dr. Sarah Lopez.