The BioCE team at LCBM and the I2BM team at DCM are focusing on the development of artificial metalloenzymes as catalysts for oxidation reactions. Artificial metalloenzymes are catalysts resulting from the merging of a biomolecule, in charge of the selectivity, and a bioinorganic complex, in charge of the reactivity. Previous studies have shown that the combination of the NikA protein with iron complexes allowed the oxidation of alkenes and thioethers but with very low enantiomeric excesses.
In this study, the protein was replaced by a G-quadruplex oligonucleotide, stabilized in a parallel topology constrained by its immobilization on a peptide scaffold. It was found that the interaction between a bipyridine copper complex and the oligonucleotide allowed reaching an enantiomeric excess up to 73 % during the catalytic oxidation by hydrogen peroxide of thioanisole derivatives. Modification of the 3’ and 5’-tetrads and comparison with the natural G-quadruplex allowed to identify the few reaction sites in the artificial enzyme structure and to propose a mechanism for the controlled reaction.
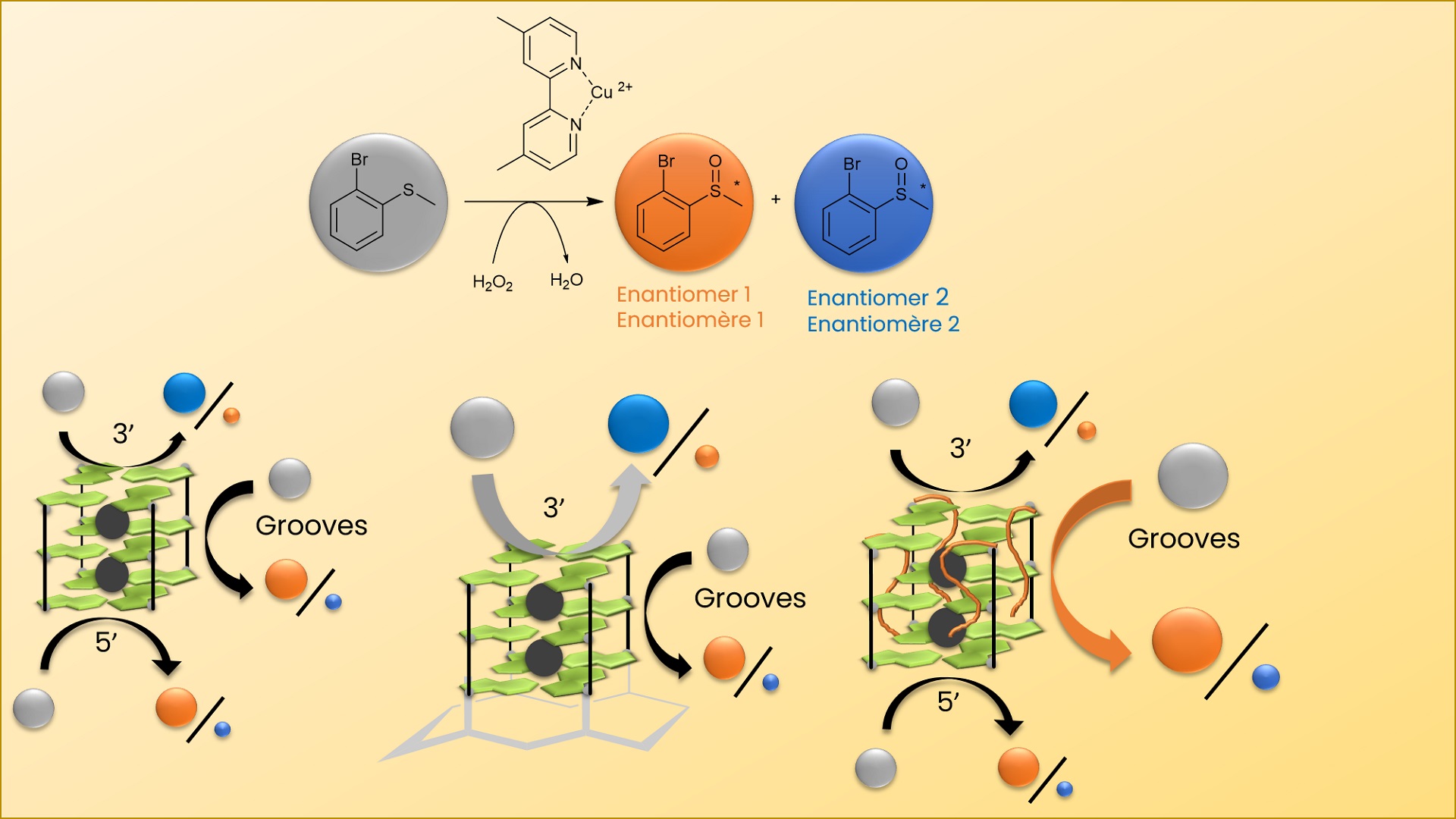
In summary, the results obtained help to decipher the enantioselective control of the sulfoxidation reaction with G-quadruplex type catalysts, highlighting the importance of the nature of additional nucleosides over the 3’-tetrad.
This research was supported by the ANR CoolCat project.