In the race for clean energy, the development of processes to produce hydrogen
via renewable resources such as water and solar energy is a highly attractive solution. Yet hydrogen production remains a costly process, due in particular to the use of platinum, a rare metal used as a catalyst in membrane electrolysis systems. Now, in a natural environment, for example in bacteria, certain enzymes act as a catalyst in the production of dihydrogen. These include hydrogenases [NiFe], which achieve high efficiency under mild conditions, and require metals as abundant as nickel or iron.
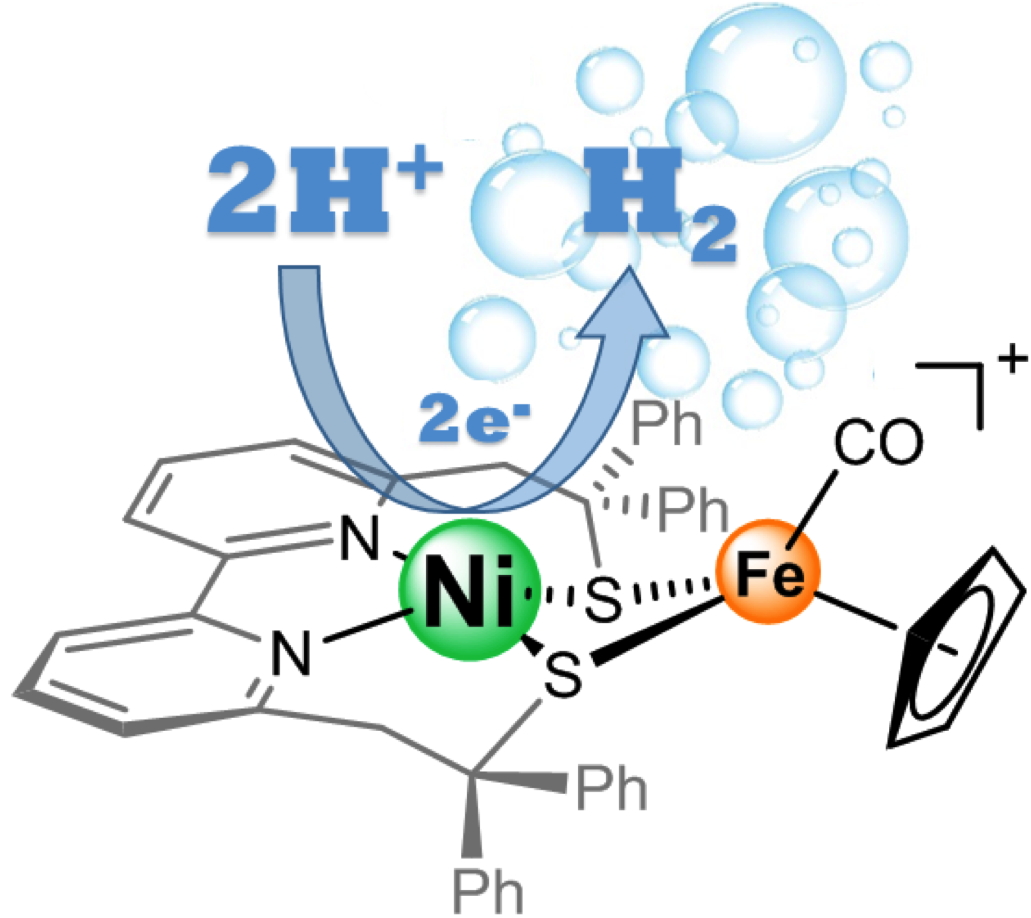
Reproducing the key elements of the active site of these enzymes within a synthetic molecule makes it possible to develop new types of catalysts without requiring noble metals. However, the nickel-iron biomimetic models described thus far do not reflect the nickel-ion-centered reactivity of the active site of these enzymes, as is the case in nature. This explains their limited performances. In this context, in collaboration with peers from Grenoble Alpes University and Aix-Marseille University, researchers at our laboratory has developed a model that relocates reactivity to the nickel center through the use of a bipyridine-dithiolate ligand. This new model is close to the activity of the enzyme as it involves two catalytic intermediates reproducing the structural and electronic characteristics of two states (Ni-L and Ni-R) of the enzymatic cycle. "The rate of hydrogen production catalyzed by this original compound clearly indicates the synergy between nickel and iron ions for hydrogen production," said Vincent Artero, researcher at our laboratory. A comparative evaluation of the performances of the new nickel-iron catalyst indicates that they significantly exceed the performances of other biomimetic models involving the coupling of two metals.