An artificial metalloenzyme (ArM) is a hybrid construct, resulting of the insertion of an inorganic complex within a biomolecule of DNA or protein type. The inorganic complex is chosen in relation to the targeted reaction. The combination forms a catalyst which is recommended for use towards eco-compatible synthetic methods and which operates under mild conditions in accordance with the principles of green chemistry.
Researchers at our laboratory designs ArMs with the aim of proposing new catalytic methodologies for oxidation reactions. In this context, this team has developed several powerful systems based in particular on the NikA protein (a nickel transport protein without enzymatic properties) and iron complexes (a non-toxic physiological metal constructs). ArMs can also be created from non-physiological metals and used to achieve reactions that native enzymes do not know how to perform.
To illustrate this theme, the researchers, in collaboration with Prof. Burzlaff, (Friedrich Alexander University, Germany), chose to work with a ruthenium complex. This complex is a catalyst for alkenes epoxidation in an organic medium whose lack of efficiency is due to its rapid degradation in the reaction medium. In order to increase the stability, they associated it with the NikA protein. They then fully structurally characterized this first Ru-based hybrid, and then determined its catalytic properties. This new ArM shown extremely interesting properties since, in addition to the expected stabilization, the activation of the complex by the protein has led unexpectedly to the selective and specific production of chlorohydrins (
Figure) in place of the expected epoxides.
This ArM therefore has a unique and never described reactivity in artificial and native enzymes. In addition, and unlike a native enzyme, this ArM accepts a wide range of substrates with a more or less activated double bond and a variable steric hindrance, potentially offering a greater number of applications.
Researchers now aim to make this chemical reaction attractive from an industrial point of view and to propose a totally eco-compatible catalyst by modifying the oxidant used, a still too noxious substance.
This study illustrates the power of the combination of a protein and a synthetic catalytic core and illustrates the potential of nanoscience in the field of biocatalysis.
This work gathers studies of synthesis and catalysis, crystallography, biochemistry and molecular modeling and results from a collaboration between four teams of the Chemistry and Biology of Metals laboratory: BioCE, BioCat, BioMet and MCT. It is funded by the ANR CrystalBall and a thesis grant from Labex ARCANE.
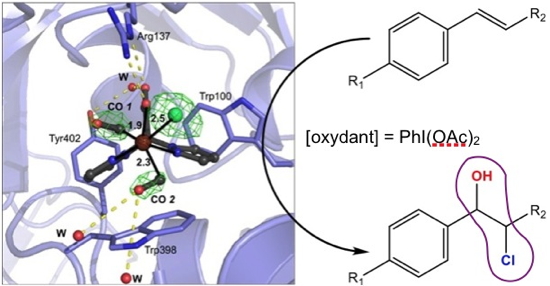
The coordination of the complex within the protein via supramolecular interactions causes the departure of one of the CO groups, observed by X-ray diffraction, making possible the entry of the oxidant into the coordination sphere of the metal and therefore the activating of the oxidant. In this image, we can see the CO 2 group about to be released.
In purple the chlorhydrin motif.