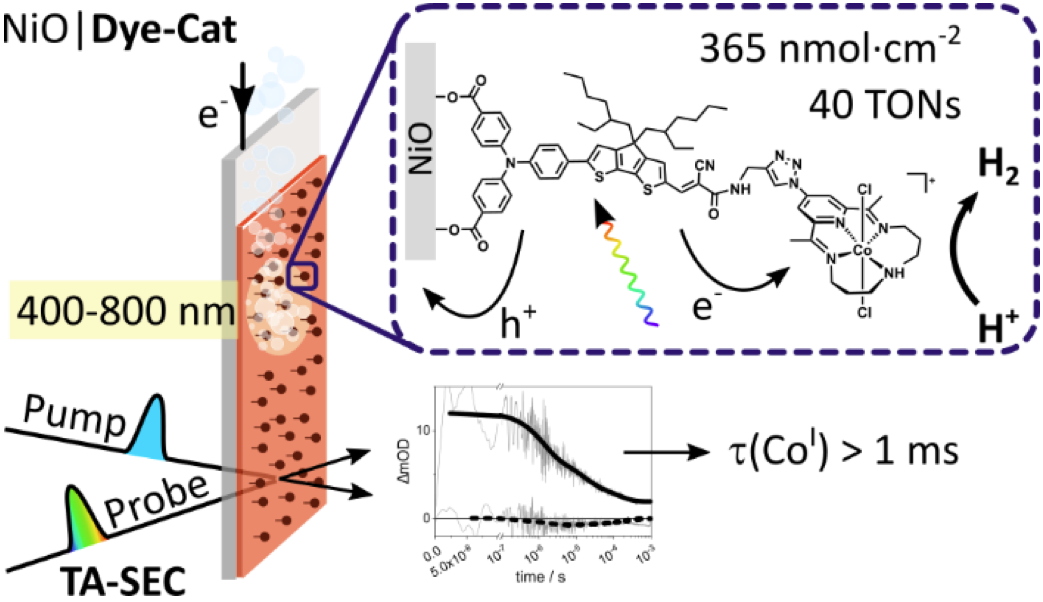
In this study, structure-activity relationships demonstrated the value of working with a robust cobalt tetraazamacrocyclic catalyst in aqueous media. The resulting photocathode efficiently produces hydrogen for 4 hours with a faradaic efficiency of 70%, placing it among the best performing examples to date. However, photocurrents rarely exceed 10 µA.cm
-2 and decrease rapidly over time. In order to identify the parameters limiting the photoelectrocatalytic activity and the stability of these molecular photocathodes, we relied on a unique combination of spectroscopic studies carried out under
operando and
post-operando conditions. Although the formation of the catalytically active Co(I) species was observed at the electrode surface, the lack of electron transfer efficiency to the catalyst and the intrinsically associated degradation of the organic dye were identified as the main barriers limiting the performance of this photoelectrocatalytic system. This work therefore paves the way for a more rational design of molecular photocathodes for solar fuel production and represents a further step towards the development of sustainable processes for the production of hydrogen from solar and water.
This study was supported by IDEX UGA in the framework of an "International Strategic Partnerships" project (financing of S. Bold's thesis in cotutelle with Friedrich Schiller University Jena).