Using dioxygen (O
2) for reactivity can be approached through mimicking processes catalyzed by living organisms. This is part of the so-called bio-inspired approach. O
2 is indeed a key molecule for metabolic functions. Due to its electronic configuration (He(σ
s)
2(σ
s*)
2(σ
z)
2(π
x)
2(π
x)
2(π
x*)
1(π
y*)
1), its fundamental triplet state (
3O
2) is not reactive with any organic molecule whose fundamental state is singlet (
1S). This spin-forbidden reaction is lifted in the presence of a metallic cofactor, such as iron or copper, presents at the active sites of certain metalloenzymes. Various activities ranging from O
2 transport to the activation of highly energetic C-H bonds and the biosynthesis of molecules of interest then become possible.
O
2 reduction can also simply undergo a 2e
-/2H
+ process to produce hydrogen peroxide (H
2O
2, Eq. 1) or 4e
-/4H
+ to form water (H
2O, Eq. 2)). These two reactions are part of the oxygen reduction reactions (ORR)
[1, 2].
O
2 + 2e
- + 2H
+ ⇆ H
2O
2
Eq.1

O
2 + 4e
- +2 H
+ ⇆ 2 H
2O

Eq. 2
On one hand, the 4e
- reduction is crucial for living organisms and occurs at Photosystem II (PS II). It is also the cathodic reaction in hydrogen-based fuel cells (Figure 1).
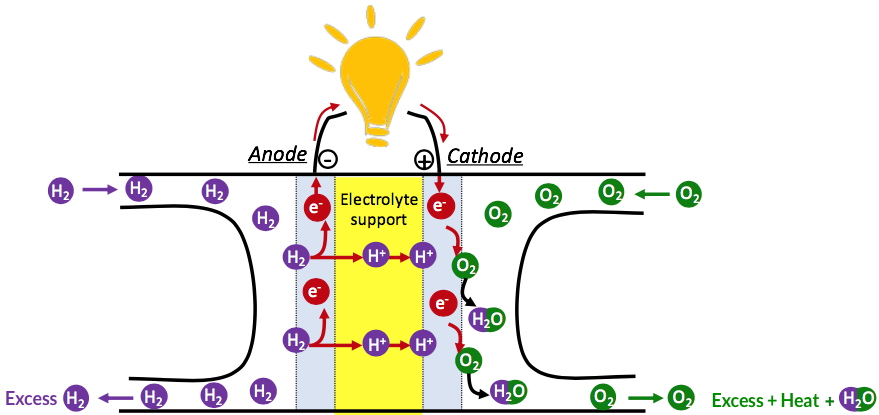 Figure 1
Figure 1. Synthetic scheme for an operating hydrogen-based fuel cell.
On the other hand, H
2O
2 is an important molecule that participates for instance in the immune response to kill microbes
[3] and as a chemical co-factor for metalloezymes such as Galactose oxidase
[4]. H
2O
2 is also a staple molecule from an industrial point of view with a worldwide demand of about 3 million tons per year
[5, 6]. It has wide applications such as oxidant in chemical reactions, paper bleaching, as antiseptic, and in the etching of electronic circuits
[7]. Recently, H
2O
2 has also emerged as a potential fuel for fuel cells since it can be either oxidized or reduced. The advantage here is that the system does not need any compartmentalization (Figure 2)
[8].
Figure 2. Scheme for a H2O2-based mono-compartmental fuel cell.
Up to now, the main H
2O
2 production relies on the energy-demanding anthraquinone process
[7] that suffers severe limitations in term of wastes treatment, efficiency or H
2O
2-content control.
There is thus a crucial need in proposing new eco-aware, sustainable and reliable production methods.
Given their structures, our mixed-valent dicopper complexes are good candidates for targeting ORR. They were tested in organic solvent and at room temperature. In these experiments, sacrificial protons and electrons were provided by the mean of the organic acid LutHBF
4 and ferrocene derivatives (MexFc), respectively. The latter are suitable here since their standard redox potentials are adjustable depending on the substitution of the cyclopentadienyl rings.
Overall, a selectivity close to 100 % in H2O2 was obtained for the MV complex
1 in acetonitrile with a careful control of the MexFc concentration (Figure 3).
[9] The ORR activity of the others members of the series are currently under investigations. These preliminary results are encouraging since they demonstrate the ability of copper ions within a N/S environment to promote almost quantitative H
2O
2 production.
However, the main drawback resides in the fact that the H
2O
2 produced is polluted by the large amount of the oxidized MexFc and therefore remains unusable.
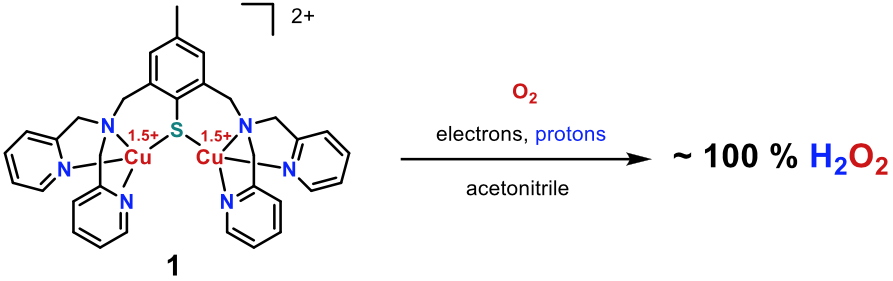 Figure 3
Figure 3.
To circumvent this problem, a new strategy based on the use of heterogeneous catalysis is currently under development in our team with a collaboration with Pr L. Bonneviot and Dr B. Albela from the ENS Lyon. In this approach, the catalyst and the electron source are co-grafted at the surface of a multi-addressable material such as mesoporous silica (denoted SiO2 from now on) to form a new hybrid. This way, they will be easily removed from the reactive medium (Figure 4), opening valuable issues in terms of sustainability and recyclability.
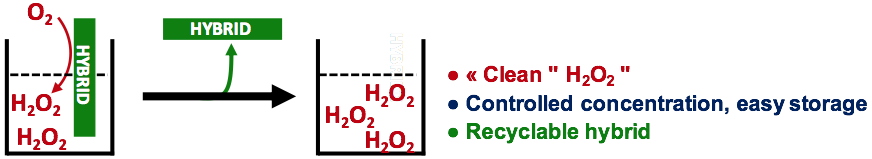
Figure 4. Approach for the use of a hybrid for clean, controlled and recyclable H
2O
2 production.
When looking closer at the structure of the targeted material (Figure 5), mesoporous SiO
2 acts as a combination of micro reactors from which a controlled reactivity and a confinement effect are expected.
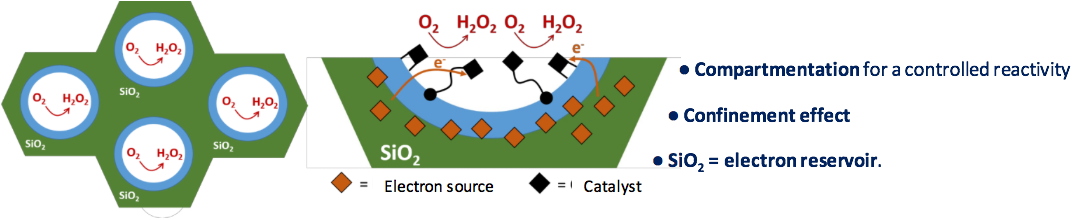
Figure 5. General strategy for the association of a molecular catalyst and MexFc sources into mesoporous SiO
2 for a controlled H
2O
2 production.
These results were published in the following article:
• Mangue, J.; Gondre, C.; Pécaut, J.; Duboc, C.; Ménage, S.; Torelli, S.;
Chem. Commun.
2020, 56, 9636-9639
(a) Machan, C. W.,
ACS Catalysis 2020, 10 (4), 2640-2655
[2] Pegis, M. L.; Wise, C. F.; Martin, D. J.; Mayer, J. M.,
Chem.Rev.
2018, 118 (5), 2340-2391
[3] Nathan, C.; Cunningham-Bussel, A.,
Nature Reviews Immunology 2013, 13 (5), 349-361
[4] Solomon, E. I.; Sundaram, U. M.; Machonkin, T. E.,
Chem. Rev.
1996, 96 (7), 2563-2605
[5] In
Ullmann's Encyclopedia of Industrial Chemistry, Wiley: 1999-2013
[6] Bryliakov, K. P.,
Chem. Rev.
2017, 117 (17), 11406-1145
[7] Campos-Martin, J. M.; Blanco-Brieva, G.; Fierro, J. L. G.,
Angew. Chem. Int. Ed.
2006, 45 (42), 6962-6984
[8] Yamada, Y.; Fukunishi, Y.; Yamazaki, S.-i.; Fukuzumi, S.,
Chem. Commun.
2010, 46 (39), 7334-7336
[9] Mangue, J.; Gondre, C.; Pécaut, J.; Duboc, C.; Ménage, S.; Torelli, S.;
Chem. Commun.
2020, 56, 9636-9639